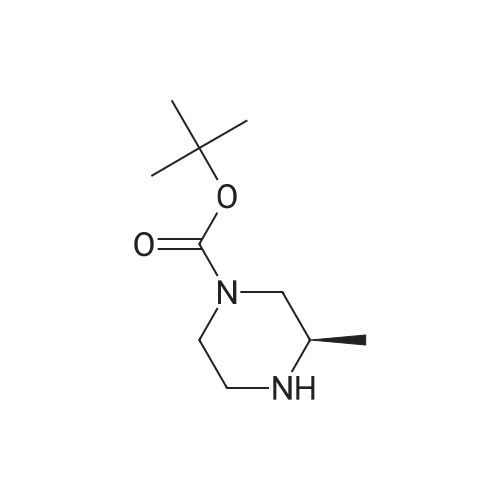
Alternatived Products of [ 147081-29-6 ]
Product Details of [ 147081-29-6 ]
| CAS No. : | 147081-29-6 |
MDL No. : | MFCD02683204 |
| Formula : |
C10H20N2O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | FMLPQHJYUZTHQS-QMMMGPOBSA-N |
| M.W : |
200.28
|
Pubchem ID : | 7023035 |
| Synonyms : |
|
Chemical Name : | (S)-tert-Butyl 3-methylpiperazine-1-carboxylate |
Safety of [ 147081-29-6 ]
Application In Synthesis of [ 147081-29-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 147081-29-6 ]
- 1
-
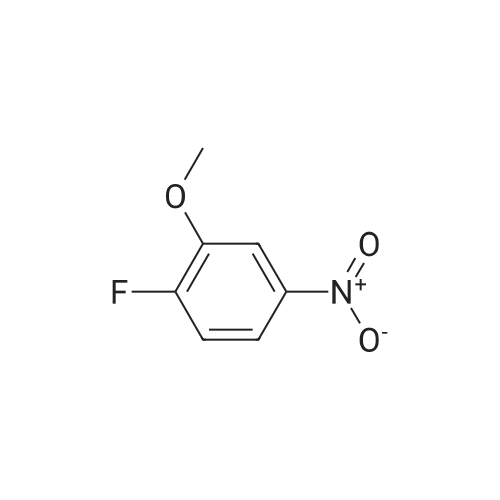 [ 454-16-0 ]
[ 454-16-0 ]

-
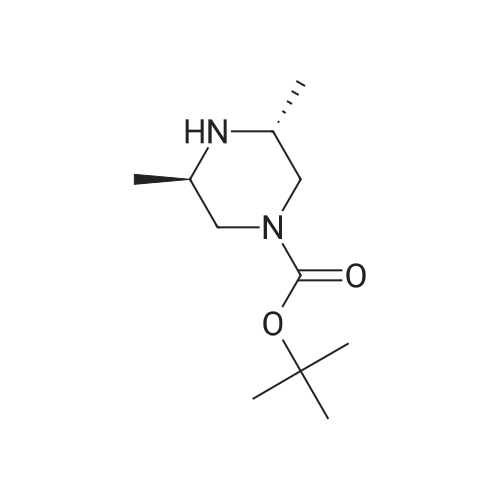
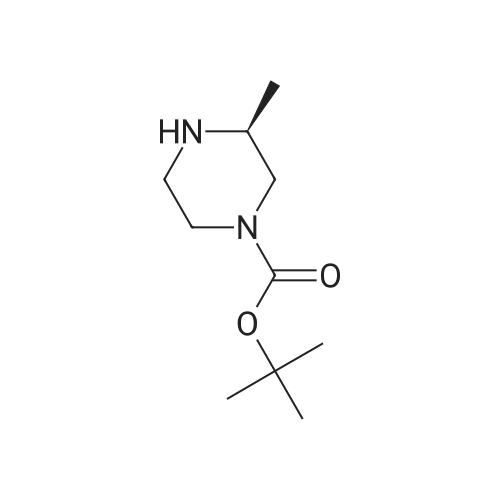 [ 147081-29-6 ]
[ 147081-29-6 ]

-
(S)-tert-butyl 4-(2-methoxy-4-nitrophenyl)-3-methylpiperazine-1-carboxylate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With potassium carbonate; In 1-methyl-pyrrolidin-2-one; at 100℃; |
1-Fluoro-2-methoxy-4-nitrobenzene (2000 mg, 11.69 mmol), (S)-tert-butyl 3-methylpiperazine-1-carboxylate (2340.71 mg, 11.69 mmol), potassium carbonate (4845.75 mg, 35.06 mmol) in NMP (18 ml) was stirred at 100° C. for 3 overnights. The reaction was diluted with EtOAc, washed with water, dried and concentrated. The crude was washed with EtOAc, filtered and dried to provide (S)-tert-butyl 4-(2-methoxy-4-nitrophenyl)-3-methylpiperazine-1-carboxylate. |
- 2
-
 [ 454-16-0 ]
[ 454-16-0 ]

-
 [ 147081-29-6 ]
[ 147081-29-6 ]

-
(S)-tert-butyl 4-(4-((4-chloro-1,3,5-triazin-2-yl)amino)-2-methoxyphenyl)-3-methylpiperazine-1-carboxylate
[ No CAS ]
- 3
-
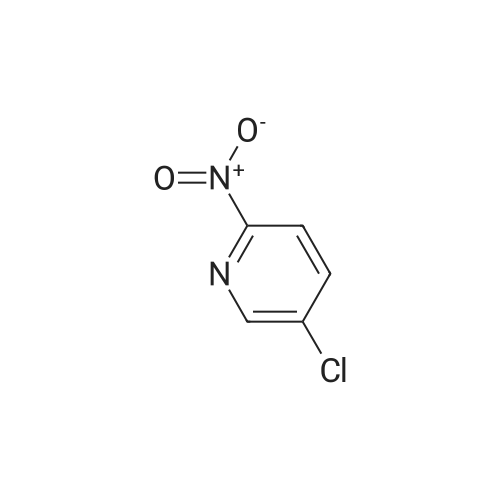 [ 52092-47-4 ]
[ 52092-47-4 ]

-
 [ 147081-29-6 ]
[ 147081-29-6 ]

-
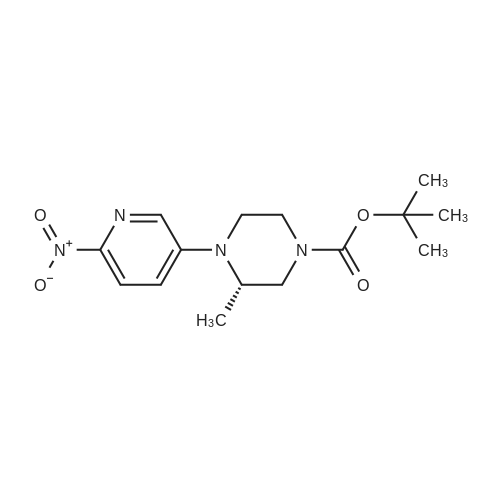 [ 1433849-53-6 ]
[ 1433849-53-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 80.5% |
With potassium phosphate; palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In 1,4-dioxane; at 95 - 105℃;Inert atmosphere; |
[0232] In a second step, compound 154 was prepared from compounds 40 and 50 as follows: (0334) (0335) [0233] Dioxane (1.5 L, 10 v/wpercent) was charged to a reaction flask and agitation was started. The reaction flask was evacuated and refilled with N2 three times. Compound 50 (118.7 g, 733.7 mmol, 1.02 eq.), compound 40 (150 g, 718 mmol, 1.0 eq.), and K3P04 (318 g, 1468 mmol, 2.09 eq.) were charged to the reaction flask with constant flow of N2. The reaction flask was evacuated and refilled with N2 three times. Pd(OAc)2 (3.4 g, 15.1 mmol, 0.021 eq.) catalyst and BINAP ligand (9.3 g, 14.9 mmol, 0.021 eq) were added to the reaction flask with constant flow of N2. The reaction flask was evacuated and refilled with N2 three times and N2 flow was continued for 1 h. The mixture was heated to 95 to 105°C and stirred at temperature for 15 h under N2 flow. The reacted mixture was cooled to 50 to 60°C and filtered at that temperature. The collected solids were washed with hot dioxane. The liquid filtrate was concentrated to dryness in vacuo at 50 to 60°C to form a residue, z'-propanol (300 g) was combined with the residue and the mixture was slurried at -5 to 5°C for 1 hour and then filtered. The collected solids were washed with cold z'-propanol. The wet solids were dried in vacuo at 60 to 70°C to yield compound 154 (t-butyl (S)-3-methyl-4-(6-nitropyridin- 3-yl)piperazine-l-carboxylate). The purity of compound 154 was 99.5 areapercent by HPLC, the assay was 94.4percent and the yield was 80.5percent. [0234] |
- 4
-
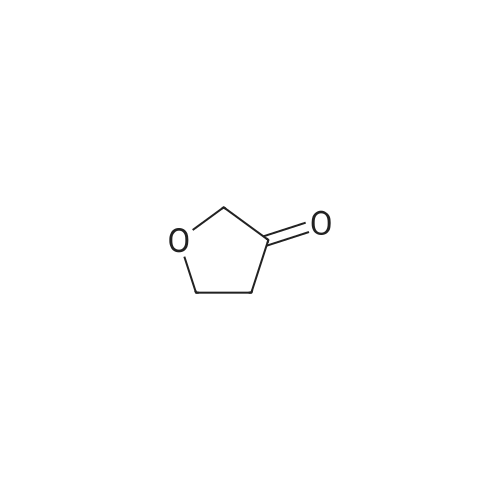 [ 22929-52-8 ]
[ 22929-52-8 ]

-
 [ 147081-29-6 ]
[ 147081-29-6 ]

-
tert-butyl (3S)-3-methyl-4-(tetrahydrofuran-3-yl)piperazine-1-carboxylate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
(S)-Tert-butyl 3-methylpiperazine-l-carboxylate (601 mg, 3 mmol) and <strong>[22929-52-8]dihydrofuran-3(2H)-one</strong> (501 mg, 5.82 mmol) were dissolved in DCE (12 mL). 4A Molecular Sieves (1000 mg, powdered and dried) were added and the mixture was stirred for 60 minutes. Sodium (0488) triacetoxyborohydride (1208 mg, 5.70 mmol) was then added portionwise over -10 minutes. The reaction was allowed to stir overnight. The reaction was filtered and the solid was washed with DCM, partitioned between aq. NaHC03 and DCM, then the separated organic fraction was dried over sodium sulfate, filtered and evaporated. The residue was purified by silica gel chromatography, eluting with a gradient of 3:1 EtOAc:EtOH in hexanes, and dried in vacuo overnight to give target product. MS[M+H]+: 271. |
- 5
-
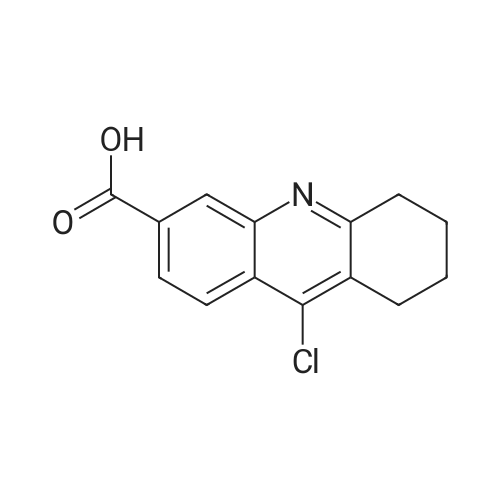 [ 902586-59-8 ]
[ 902586-59-8 ]

-
 [ 147081-29-6 ]
[ 147081-29-6 ]

-
propyl (S)-4-(9-chloro-5,6,7,8-tetrahydroacridine-3-carbonyl)-3-methylpiperazine-1-carboxylate
[ No CAS ]
- 6
-
 [ 902586-59-8 ]
[ 902586-59-8 ]

-
 [ 147081-29-6 ]
[ 147081-29-6 ]

-
tert-butyl 4-(9-chloro-5,6,7,8-tetrahydroacridine-3-carbonyl)-3-methylpiperazine-1-carboxylate
[ No CAS ]
- 7
-
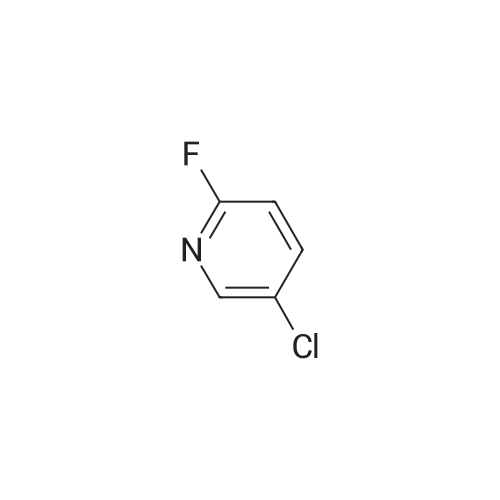 [ 1480-65-5 ]
[ 1480-65-5 ]

-
 [ 147081-29-6 ]
[ 147081-29-6 ]

-
(S)-tert-butyl 4-(5-chloropyridin-2-yl)-3-methylpiperazine-1-carboxylate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 85.74% |
With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; lithium hexamethyldisilazane; at 90℃; for 16h;Inert atmosphere; |
To stirred (S)-tert-butyl 3-methylpiperazine-1 -carboxylate (18 g, 90 mmol, 1 eq) was added <strong>[1480-65-5]5-chloro-2-fluoropyridine</strong> (23.58 g, 180 mmol, 2 eq), xantphos (1.56 g, 2.7 mmol, 0.03 eq), Pd2(dba)3 (2.47 g, 2.7 mmol, 0.03 eq) and Li- HMDS (450 mL, 450 mmol, 5 eq) at RT under an argon atmosphere, then the reaction mixture was heated to 90C for 16 h. TLC analysis indicated formation of a less polar spot. The reaction mixture was cooled to RT then filtered through a celite pad, which was washed with EtOAc (3 times). The filtrate was diluted with water and extracted with EtOAc (3x100 mL). The combined organic layer was dried over Na2S04 then concentrated to crude compound. The crude compound was purified by column chromatography (silica gel, 100-200 mesh) using 0-10% EtOAc in petroleum ether as eluent to afford (S)-tert-butyl 4-(5-chloropyridin-2-yl)-3- methylpiperazine-1 -carboxylate (24 g, 85.74%) as a brown oil. LC-MS: m/z 312.17 (M + H). |
| 85.74% |
With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; lithium hexamethyldisilazane; at 90℃; for 16h;Inert atmosphere; |
To a stirred compound 2 (18g, 90mmol, 1 eq) was added compound 3 (23.58g, 180mmol, 2eq), xantphos (1.56g, 2.7mmol, 0.03eq), Pd2(dba)3 (2.47g, 2.7mmol, 0.03eq) and Li-HMDS (450ml_, 450mmol, 5eq) at RT under argon atmosphere. Then, the reaction mixture was heated to 90C for 16h. TLC analysis indicated formation of a less polar spot. The reaction mixture was cooled to RT then filtered through celite pad, which was washed with EtOAc (3 times). The filtrate was diluted with water and extracted with EtOAc (3X100ml_). The combined organic layer was dried over Na2S04 then concentrated to crude compound. The crude compound was purified by column chromatography (silica gel, 100-200 mesh) using 0-10% EtOAc in petroleum ether as eluent to afford compound 4 (24g, 85.74%) as a brown oil. LC-MS: m/z 312.17 (M + H); |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping