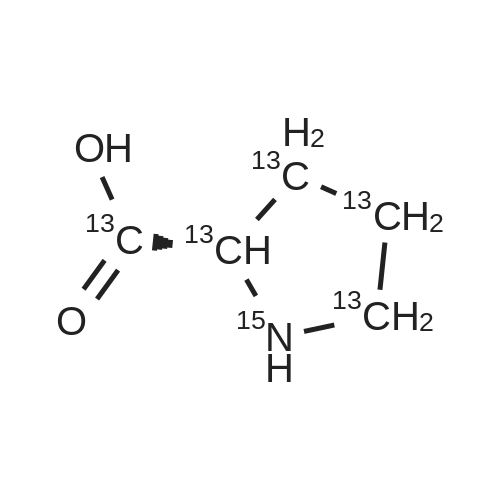
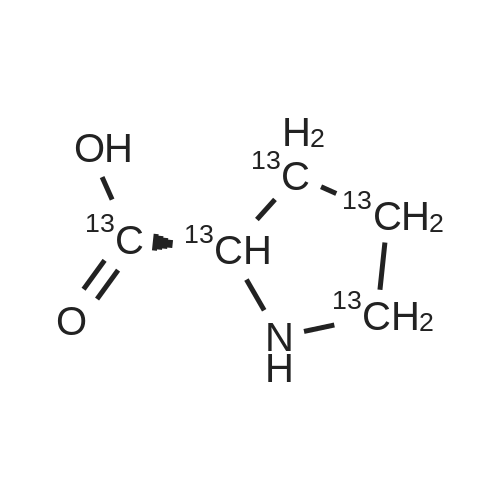
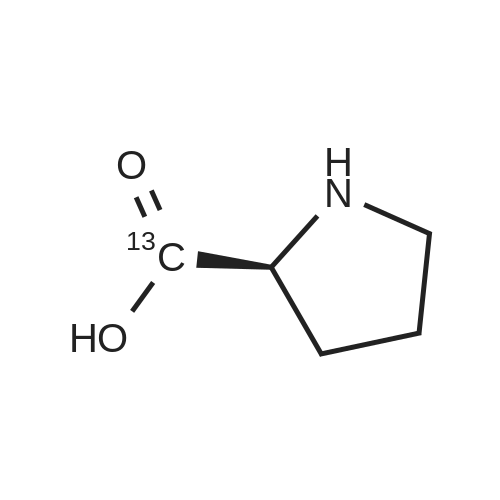
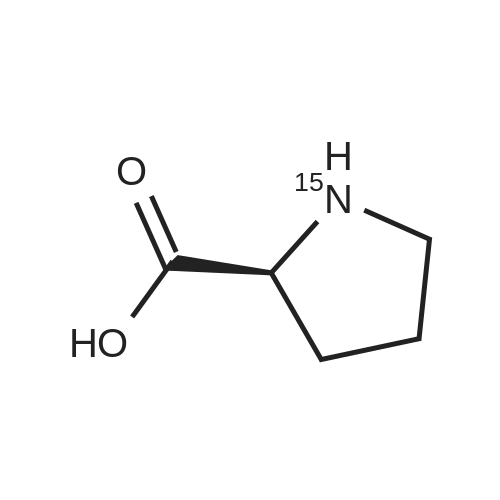
Chromatographic separation by RPLC-ESI-MS of all hydroxyproline isomers for the characterization of collagens from different sources
Martina Lioi
;
Sara Tengattini
;
Roberto Gotti
, et al.
J. Chromatogr. A,2024,464771.
DOI:
10.1016/j.chroma.2024.464771
More
Abstract: During collagen biosynthesis, proline is post-translationally converted to hydroxyproline by specific enzymes. This amino acid, unique to collagen, plays a crucial role in stabilizing the collagen triple helix structure and could serve as an important biomarker for collagen content and quality analysis. Hydroxyproline has four isomers, depending on whether proline is hydroxylated at position 4 or 3 and on whether the cis- or trans- conformation is formed. Moreover, as extensive hydrolysis of collagen is required for its amino acid analysis, epimerization may also occur, although to a lesser extent, giving a total of eight possible isomers.
The aim of the present study was to develop a reversed-phase high-performance liquid chromatography-UV-mass spectrometry (RPLC-UV-MS) method for the separation and quantification of all eight hydroxyproline isomers. After the chiral derivatization of the hydroxyproline isomers with Nα-(2,4-dinitro-5-fluorophenyl)-L-valinamide (L-FDVA), to enable their UV detection, the derivatized diastereoisomers were separated by testing different C18 column technologies and morphologies and optimizing operative conditions such as the mobile phase composition (solvent, additives), elution mode, flow rate and temperature. Baseline resolution of all eight isomers was achieved on a HALO? ES-C18 reversed-phase column (150×1.5 mm, 2.7 μm, 160 ?) using isocratic elution and MS-compatible mobile phase.
The optimized method was validated for the quantification of hydroxyproline isomers and then applied to different collagen hydrolysates to gain insight and a deeper understanding of hydroxyproline abundances in different species (human, chicken) and sources (native, recombinant).
Keywords:
Collagen ;
Amino acid analysis ;
Hydroxyproline isomers ;
Recombinant collagen ;
Reverse phase chromatography ;
Mass spectrometry
Purchased from AmBeed:
147-85-3 ;
73-32-5 ;
61-90-5 ;
51-35-4

Synthesis of (2 S, 3 R, 4 R)-Dihydroxyisoleucine for Use in Amatoxin Synthesis
Chandra, Shambhu Deo
;
Gunasekera, Shanal
;
Noichl, Benjamin Philipp
, et al.
JOC,2024,89(17):12739-12747.
DOI:
10.1021/acs.joc.4c01051
More
Abstract: We report a streamlined synthesis of (2S,3R,4R)-4,5-dihydroxy isoleucine (DHIle), an amino acid found in α-amanitin, which appears to be critical for toxicity. This synthetic route is transition metal-free and enables the production of significant quantities of DHIle with suitable protection for use in peptide synthesis. Its incorporation into a cytotoxic amatoxin analog is reported.
Purchased from AmBeed:
147-85-3 ;
74124-79-1 ;
3001-72-7 ;
288-32-4

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping



-Dihydroxyisoleucine for Use in Amatoxin Synthesis.png)
 and (4+2) Cycloaddition Reactions of Sulfamidate Imine-Derived Azadienes Synthesis of Spirocyclic Pyrrolidines and Tetrahydroquinolines.png)