| 72% |
|
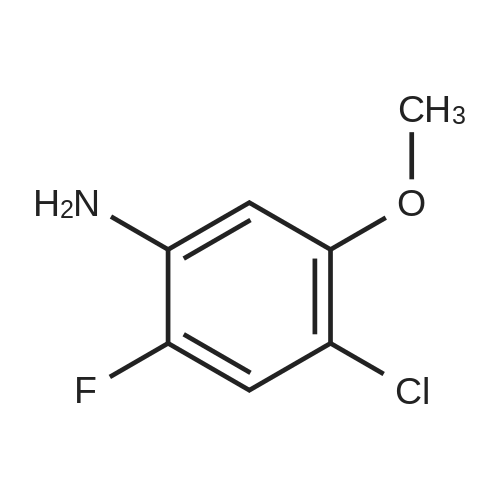
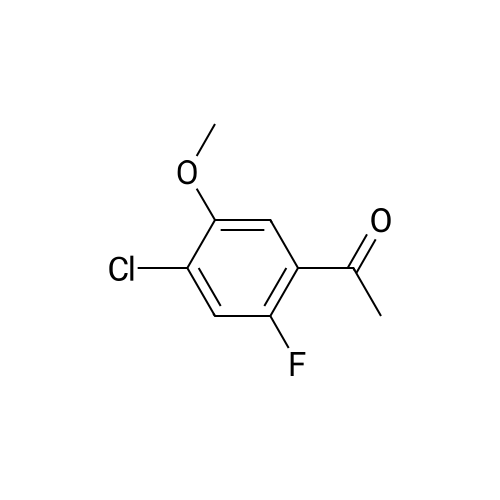
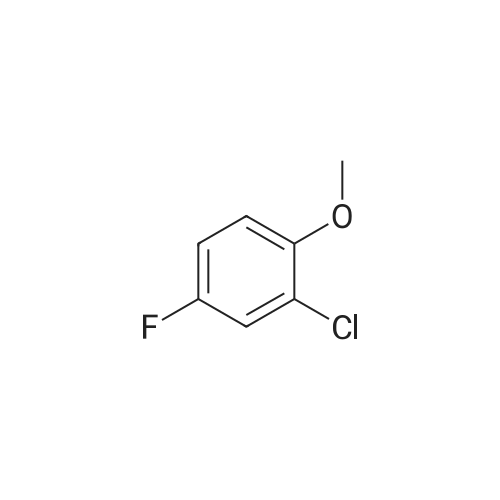
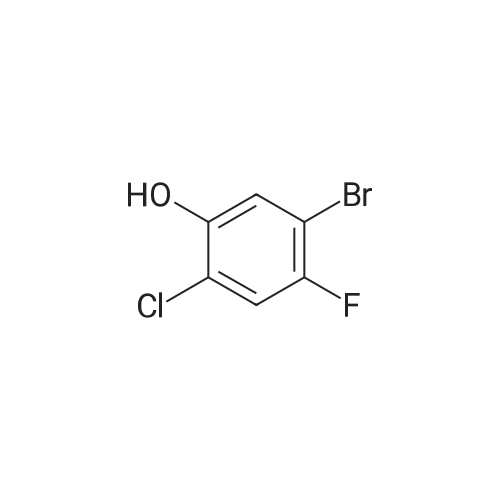
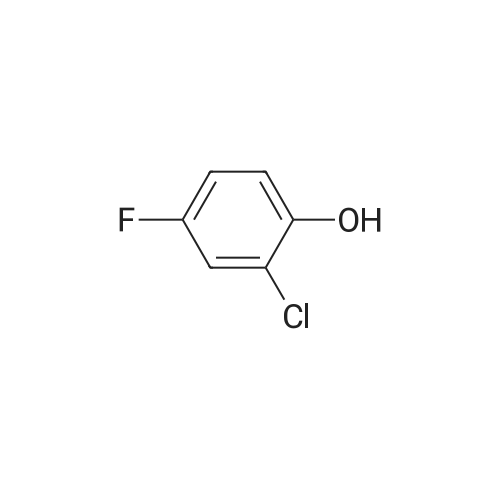
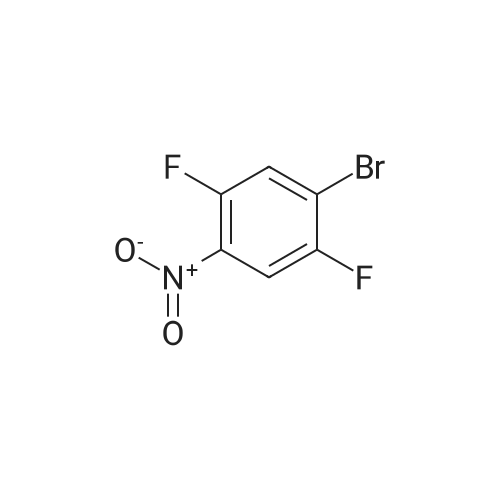
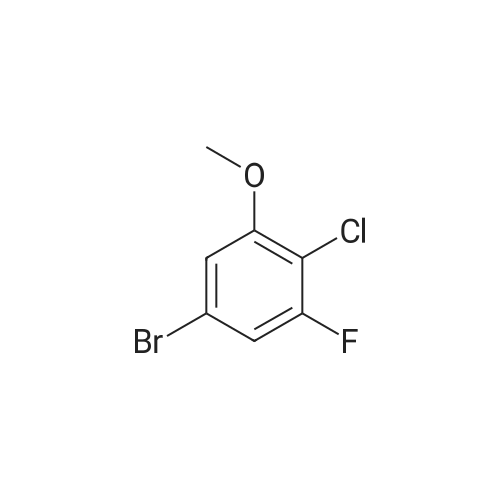
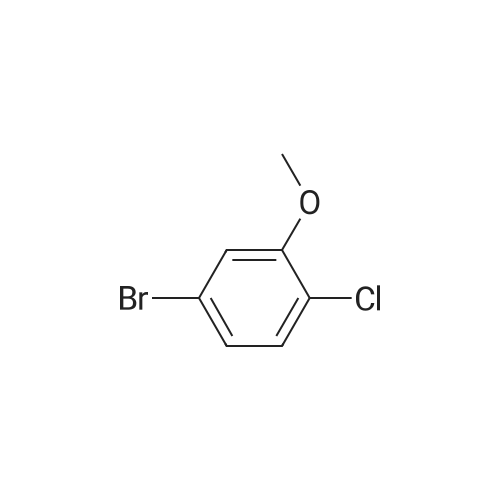
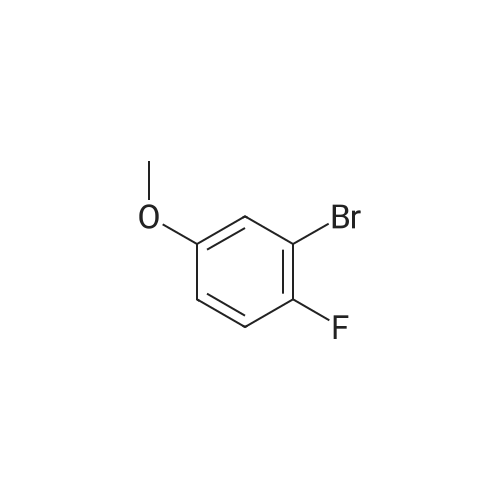
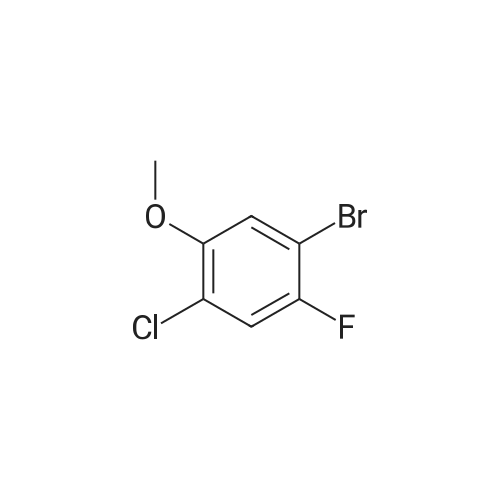
To THF (40mL) solution of5-bromo-2-chloro-4-fluoro anisole (9.23g, 38.5mmol), isopropyl magnesium chloride solution(20.2mL, 2M-THF solution) was added at -40 ,and was stirred at room temperature for 30 minutes. THFsolution of the obtained Grignard reagent was dropped at -40 toTHF (5mL) solution of diethyl oxalate (5.06mL, 36.6mmol),and the mixture was stirred for one hour at 0 .Saturated ammonium chloride solution (50mL)was added to the reaction solution, and extracted with ethyl acetate (100mL ×3). The organic layer was dried over anhydrous magnesium sulfate, then thecrude product obtained by concentrating under reduced pressure was eluted bysilica gel column chromatography (hexane: ethyl acetate = 10: 1) and colorlessliquid of 2- (4-chloro-2-fluoro - 5-methoxyphenyl) -2-oxo ethyl acetate (7.24 g,yield: 72%) was obtained. |
| 72% |
|
<strong>[146447-18-9]5-bromo-2-chloro-4-fluoroanisole</strong> (9.23 g, 38.5 mmol) in THF (40 mL) solution of isopropylmagnesium chloride solution (20.2mL, 2M-THF solution) at -40 C It was added and stirred for 30 minutes at room temperature. Obtained mixture THF solution, diethyl oxalate (5.06mL, 36.6mmol) was dropped at -40C in THF (5mL) solution, and the mixture was stirred for one hour at 0C . Saturated ammonium chloride solution (50mL) was added to the reaction solution, and extracted with ethyl acetate (100mL × 3). The organic layer was dried over anhydrous magnesium sulfate, concentrated under reduced pressure to give crude product was purified by silica gel column chromatography -eluted with (hexane: ethyl acetate = 10: 1) ethyl-2-(4-chloro-2-fluoro-5-methoxyphenyl)-2-oxoacetate colorless liquid (7.24 g, yield: 72%) was obtained |
| 68% |
|
Reference Example-26 After THF (25 mL) was added to magnesium (2.55 g, 105 mmol) at room temperature, iodine (10 mg) was added thereto, then, a solution of <strong>[146447-18-9]5-bromo-2-chloro-4-fluoroanisole</strong> (23.9 g, 100 mmol) in THF (50 mL) was slowly added thereto, and the resultant product was stirred for 1 hour, whereby a Grignard reagent was prepared. The Grignard reagent was added dropwise to a solution of diethyl oxalate (14.5 mL, 105 mmol) in THF (14.5 mL) at -40 C. or lower. After the dropping was completed, the temperature of the reaction solution was raised to 0 C., followed by stirring 1 hour. After the reaction was completed, a saturated ammonium chloride aqueous solution (100 mL) was added to the reaction solution, and the resultant product was diluted with water (100 mL) and extracted with ethyl acetate (200 L*2). After the organic layer was dried over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure. The crude product was distilled under reduced pressure (125 C. to 130 C./4 mmHg), whereby ethyl 2-(4-chloro-2-fluoro-5-methoxyphenyl)-2-oxoacetate (17.8 g, yield: 68%) was obtained as a pale yellow oily material. 1H-NMR (400 MHz, CDCl3): delta1.40 (t, J=7.2 Hz, 3H). 3.95 (s, 3H), 4.43 (q, J=7.2 Hz, 2H), 7.25 (d, J=9.9 Hz, 1H), 7.42 (d, J=5.9 Hz, 1H). 19F-NMR (376 MHz, CDCl3): delta-119.7 (s, 1F). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping