| 88% |
With water monomer; potassium hydroxide; In tetrahydrofuran; methanol; at 100℃; for 12h; |
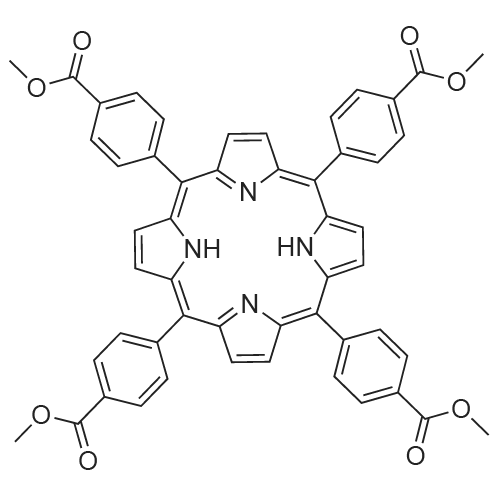
The obtained <strong>[22112-83-0]TPPCOOMe</strong> (0.25 g, 2.9mmol) was added in mixed solvent of MeOH (10 mL) andtetrahydrofuran (THF, 10 mL). Then, a solution of KOH (0.49 g, 8.7 mmol, 10 mL)was introduced. After refluxing and stirring for 12 h at 100 C, THF and MeOH in theresulting solution were evaporated. Then, additional water was added to the resultingaqueous phase. The mixture was heated until solids were completely dissolved. Thehomogeneous solution was acidified with 1 mol/L HCl until no further precipitatedetected. The green solid (0.26g, 88%) was collected by filtration, washed with waterand dried in vacuum.1H NMR (400 MHz, DMSO-d6): δ 8.82 (s, 8H), 8.33 (q, J = 8.3Hz, 16H) (Fig. S3). m/z = 790.6 (Fig. S4). |
| 80% |
With hydrogenchloride; In trifluoroacetic acid; at 85℃; for 36h; |
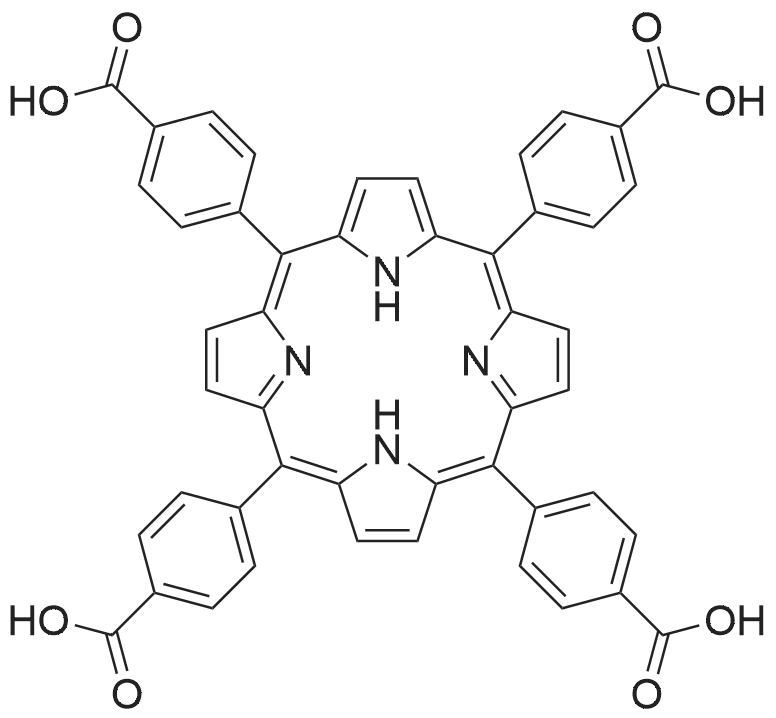
H2(<strong>[22112-83-0]TMCPP</strong>) (0.12 mmol) was dissolved in 5 ml of trifluoroacetic acid, then 2.5 mL of HCl (35%)was added to the solution, and the mixture was stirred at 85 C for 36 h. The reaction mixturewas diluted with cold water to give green precipitate, filtered, and washed with water andCH2Cl2 at three times to remove unreacted H2(<strong>[22112-83-0]TMCPP</strong>). The green solid was dissolved in 10 mLpyridine, filtered, and evaporated. After washing with water and CH2Cl2 to afford H2(TCPP) in80% yield as purple powder.H2(TCPP), UV-Vis (DMF) λmax, 422 (a Soret band), 515, 552,592 and 648 (Q bands).1H NMR (500 MHz, CDCl3): δ 13.3 (br, 4H), 8.84 (s, 8H), 8.37 (d, J=8.15 Hz, 8H), 8.33 (d, J=8.15 Hz,8H); 13C NMR (126 MHz, CDCl3) δ 119.78 (Cmeso), 128.35 (ArCmeta), 131.00 (C), 134.89(ArCortho), 145.84 (Cα), 167.90 (C=O). Elemental analysis: calculated for C48H30N4O8: C 72.90, H3.82, N 7.09. Found: C 72.11, H 3.67, N 6.75. High-resolution MS, calcd for C48H30N4O8: 790.2064.Found m/z: 790.0068. |
| 60% |
With potassium hydroxide; In tetrahydrofuran; methanol; water monomer; for 12h;Reflux; |
The obtained methyl ester (0.75g, 0.885mmol) was stirred in THF/MeOH mixed solvent (50mL, 1:1 v/v), and then a solution of aqueous KOH (2.63g, 46.95mmol in H2O 25mL) was added. The resultant mixture was stirred and refluxed for 12h. After cooling down the solution to room temperature, THF and MeOH removed under reduced pressure. Further water was added to the resulting mixture in order to dissolve the solid by heating. Afterwards, the homogeneous solution was acidified by dropwise addition of 1M HCl until no further precipitate was observed. The crystals were then collected using filtration, washed with water and left to dry in air (or in vacuum) (470mg, 0.594mmol, 60 % yield). |
|
With water monomer; potassium hydroxide; In tetrahydrofuran; at 85℃; for 12h; |
Mix 2 g of the synthesized porphyrin ester, 60 mL of THF, 60 mL of methanol, and a prepared potassium hydroxide (KOH) solution (6.8 g KOH plus 60 mL of water), and condense and reflux the mixture at 85 C for 12 h. After cooling to room temperature, adjust the pH of the product to 6~7 with 1M (mol/L) HCl solution, then wash with a large amount of water by suction, and finally vacuum dry at 90C for 12h to obtain tetrakis(4-carboxyphenyl) Porphyrin (H2TCPP). |
|
With potassium hydroxide; In tetrahydrofuran; methanol; water monomer; at 80℃; for 12h; |
Pyrrole (3.0g, 0.043mol) and methyl p-formylbenzoate (6.9g, 0.042mol) were put into a 250ml Pyrrole (3.0g, 0.043mol), methyl p-formylbenzoate (6.9g, 0.042mol) and propionic acid (100mL) were added into a three-neck flask (250mL). The reaction was conducted at 150C for 12h. After reaction, the reaction mixture was cooled to room temperature, yielding black solid. After filtration, the solid was washed with ethanol and dried in vacuum to obtain purple porphyrin ester precursor. The precursor (1.95g) was dissolved in a mixed solvent (120mL, tetrahydrofuran / methanol=1:1). 60ml 2M KOH aqueous solution was added into the mixed solvent. The mixture was heated at 80Cfor 12h. After cooling to room temperature, the mixture was acidified with 1M HCl solution until no solid was produced. Meso-tetra(4-carboxyphenyl) porphyrin was obtained through washing operation and drying treatment. Meso-tetra(4-carboxyphenyl) porphyrin (violet crystal, 32.3%). 1H NMR (DMSO-d6): δ 2.87 ppm (2H, N-H), δ 8.39 ppm (8H, phenyl), δ 8.45 ppm (8H, phenyl), δ 8.91 ppm (8H, pyrrole). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping