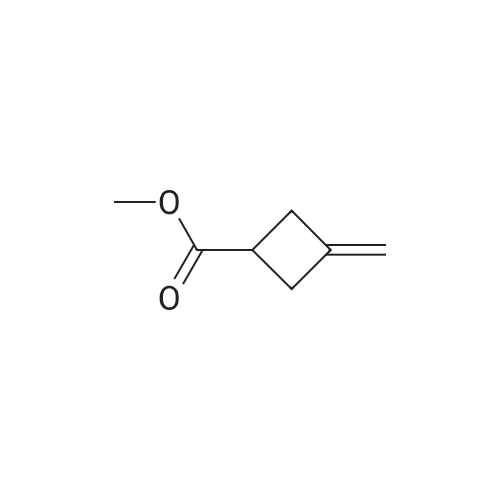
| 84% |
|
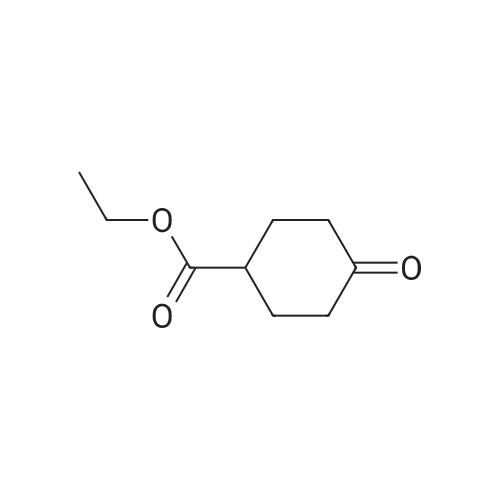
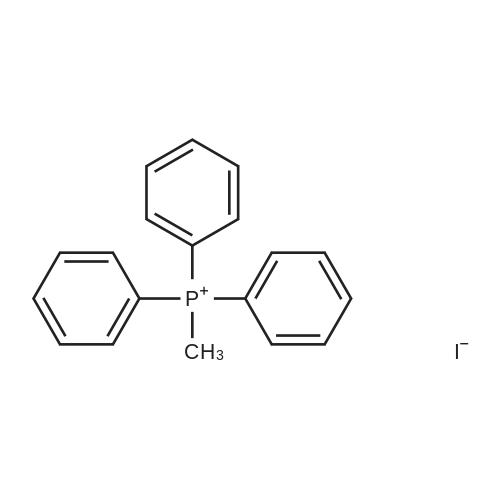
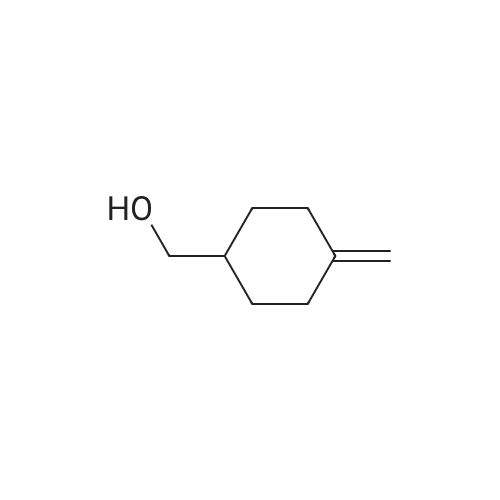
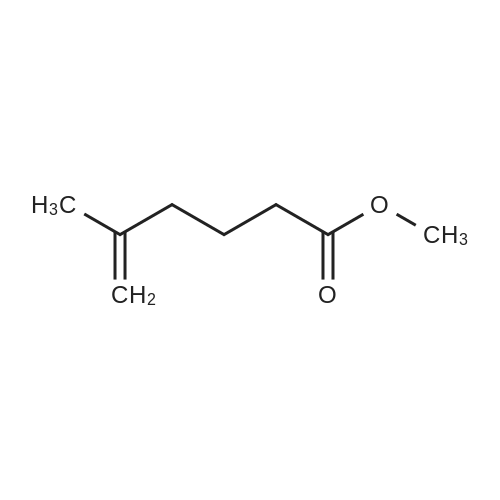
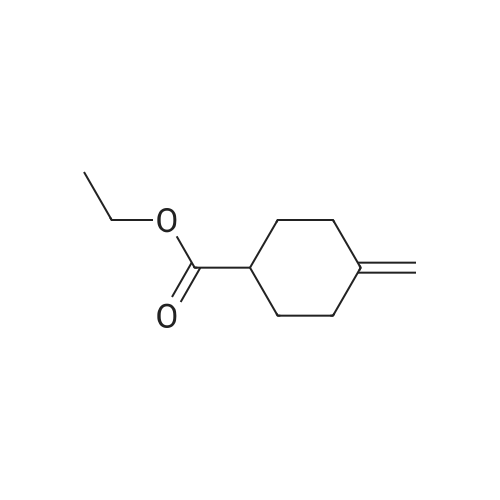
30A. Ethyl 4-methylenecyclohexanecarboxylate To a solution of (methyl)triphenylphosphonium bromide (5.18 g, 14.51 mmol) in THF (50 mL) at 0 C. was added n-butyllithium (9.07 mL, 14.51 mmol). The reaction mixture was stirred at 0 C. for 30 min. Ethyl 4-oxocyclohexanecarboxylate (1.9 g, 11.16 mmol) in THF (8 mL) was then added at 0 C. and the reaction was warmed to rt and stirred for 2 h. The reaction was quenched with sat'd aq. NH4Cl and diluted with EtOAc. The organic layer was washed with water and brine, dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash chromatography on SiO2 (0 to 50% EtOAc:hexanes) to afford the title compound (1.58 g, 84% yield) as a colorless oil. 1H NMR (500 MHz, CDCl3) delta 4.65 (s, 2H), 4.17-4.11 (m, 2H), 2.44 (tt, J=11.1, 3.8 Hz, 1H), 2.35 (dt, J=13.7, 4.0 Hz, 2H), 2.11-1.96 (m, 4H), 1.64-1.54 (m, 2H), 1.28-1.23 (m, 3H). |
| 81% |
|
Preparation of 4-Methylenecyclohexanecarboxylic acid ethyl ester; A suspension of methyl phosphonium bromide (3.15 g, 8.82 mmol) in dry THF (20 mL) was cooled to 00C, a solution of KtBuO (1.185 g, 10.58 mmol) in dry THF (15 mL) was added. The reaction mixture was allowed to rt and maintained for 1 h. The resulting mixture was cooled to 5-100C, 4-Oxo-cyclohexanecarboxylic acid ethyl ester (1.0 g, 5.88 mmol) (Chem. Abstr. Reg. No. 17159-79-4) was added over a period of 5 min, then was warmed to rt, maintained for 2h, then was heated to 50C and maintained over night additionally. The resulted reaction mass was diluted with water (200 mL) and extracted with EtOAc (200 mL). The organic layer was washed with water (50 mL), brine solution (100 mL), dried over anhydrous sodium sulfate and concentrated to obtain the crude product. The crude was purified by column chromatography over silica gel column using 3-4% EtOAc in pet ether as eluting solvent to obtain the pure product (1) as liquid (800 mg, 81%). 1HNMR(CDCI3): delta 4.65(s, 2H), 4.12(q, 2H), 2.3-2.5(m, 3H), 1.9-2.16(m, 4H), 1.5-1.7(m, 2H) and 1.25(t, 3H). Mass: (M+1) 169 calculated for Ci0H16O2. |
| 76% |
|
To a suspension of MePh3PBr (37.1 g, 104 mmol) in THF (500 mL) at 0C was added slowly LDA (1.2 eq) over 1 h. The resulting orange solution was stirred for 30 min before ethyl 4-oxocyclohexanecarboxyIate (16.1 g, 94.4 mmol) was added dropwise. The resulting suspension was warmed to rt and stirred overnight (may not necessary). A saturated NH4CI (aq) was added and THF was removed. The aqueous residue was extracted with EtOAc (100*3). The combined organic layers were washed with brine, dried over Na2S04 and concentrated. The residue was purified by passing through a short silica gel plug(hexanes/EtOAc 7:1). After being concentrated, ethyl 4-methylenecyclohexanecarboxylate was obtained as pale yellow oil (12.1 g, 76%). |
| 73% |
|
Lithium bis(trimethylsilyl)amide (1.0 M in THF, 15 mL, 15 mmol) was added dropwisely to a stirred solution of methyltriphenylphosphonium bromide (5.36 g, 15 mmol) in THF (50 mL) at 0 C and stirred for 40 min at the same temperature. A solution of ethyl 4-oxocyclohexanecarboxylate (2.04 g, 12 mmol) in THF (20 mL) was added slowly at 0 C and stirred for 2 h from 0 C to room temperature. The reaction was quenched with saturated NH4C1 aq. and extracted with hexane. The collected organic layer was dried over MgS04 and concentrated under reduced pressure. The solvent ( 100 mL, hexane/Et20 = 5/1) was added to the residue and stirred for 30 min. The suspension was filtrated. The filtrate was concentrated under reduced pressure. The residue was purified by silicagel chromatography (5% EtOAc/hexane as eluent) to provide compound A122-1 (1.478 g, 73%) as a colorless oil. |
| 68.8% |
|
N-butyllithium (21 mL, 52.5 mmol) was added dropwise to a solution of diisopropylamine (7.54 mL, 52.9 mmol) in THF (40 mL) at -78 C. over a period of 10 min. The resulted solution was stirred in an ice bath for 20 min. The above LDA solution was canulated into a suspension of methyltriphenylphosphonium bromide (19 g, 53.2 mmol) in THF (100 mL) in ice bath and the resulted mixture was stirred in the ice bath for 40 min. A solution of ethyl 4-oxocyclohexanecarboxylate (7.5 g, 44.1 mmol) in THF (20 mL) was added dropwise to this mixture. The reaction mixture was stirred for 18 h and diluted with hexane. The solid was filtered off and the filtrate was concentrated to afford a liquid. This crude product was plugged through silica gel pad (?2", EtOAc/hexane: 0 to 10%) to yield the title compound as a clear liquid (5.0 g, 68.8% yield). 1H NMR (400 MHz, CHLOROFORM-d) delta 4.76-4.60 (m, 2H), 4.20-4.08 (m, 2H), 2.45 (tt, J=11.1, 3.6 Hz, 1H), 2.35 (dt, J=13.5, 3.5 Hz, 2H), 2.14-1.96 (m, 4H), 1.67-1.52 (m, 2H), 1.30-1.22 (m, 3H) |
| 54% |
|
Methyltriphenylphosphonium bromide (4.20 g, 11.75 mmol) and potassium 2-methylpropan-2-olate (1.32 g, 11.75 mmol) were dissolved in 1,4-dioxane (20 mL) under nitrogen. The solution was stirred for 30 min then cooled to 0 C. Separately, ethyl 4-methylenecyclohexane-1-carboxylate (1.06 g, 11.75 mmol) was dissolved in 1,4-dioxane (5 mL) and added dropwise over 15 min. The reaction mixture was stirred at 20 C for 1.5 h. The volatiles were removed under reduced pressure. The crude product was dissolved in DCM (30 mL) and filtered. The filtrate was purified via silica gel chromatography eluting with 0-40% EtOAc in heptane to afford ethyl 4-methylenecyclohexane-1-carboxylate 26 (1.06 g, 54%) as a clear oil. 1H NMR (400 MHz, CDCl3) 1.25 (t, J = 7.1, 3H), 1.50 - 1.66 (m, 2H), 1.93 - 2.13 (m, 4H), 2.34 (dt, J = 12.9, 3.7, 2H), 2.43 (tt, J = 10.9, 3.6, 1H), 4.13 (q, J = 7.1, 2H), 4.64 (t, J = 1.4, 2H). 1H NMR consistent with reported literature data4 |
|
|
To a suspension of MePh3PBr (37.1 g, 104 mmol) in THF (500 mL) at 0C was added slowly LDA (1.2 eq) over 1 h. The resulting orange solution was stirred for 30 min before ethyl 4-oxocyclohexanecarboxylate (16.1 g, 94.4 mmol) was added dropwise. The resulting suspension was warmed to rt and stirred overnight. A saturated NH4CI (aq) was added and THF was removed. The aqueous residue was extracted with EtOAc (100mlx3). The combined organic layers were washed with brine, dried over Na2S04 and concentrated. The residue was purified by passing through a short silica gel plug (hexanes/EtOAc 7:1). After being concentrated, ethyl 4-methylenecyclohexanecarboxylate was obtained as a pale yellow oil (12.1 g). |
|
|
To a suspension of MePh3PBr (37.1 g, 104 mmol) in THF (500 mL) at 0 C was added slowly LDA (1.2 eq) over 1 h. The resulting orange solution was stirred for 30 min before ethyl 4-oxocyclohexanecarboxylate (16.1 g, 94.4 mmol) was added dropwise. The resulting suspension was warmed to rt and stirred overnight. A saturated NH4CI (aq) was added and THF was removed. The aqueous residue was extracted with EtOAc (100ml x 3). The combined organic layers were washed with brine, dried over Na2SC and concentrated. The residue was purified by passing through a short silica gel plug (hexanes/EtOAc 7:1). After being concentrated, ethyl 4-methylenecyclohexanecarboxyIate was obtained as a pale yellow oil (12.1 g). |
| 3.5 g |
|
Brief procedure: KO-tBu was added to a solution of methyltriphenylphosponium bromide at 0 C under nitrogen atmosphere and stirred for 30 min. To the above yellow colored reaction, a solution of ethyl 4-oxocyclohexane carboxylate in THF was added dropwise and the resulting mixture was stirred at the same temperature for 16 h. Work up: The reaction mixture was quenched with water and extracted with diethyl ether. The combined ethereal extract was dried and concentrated under reduced pressure. Purification: The crude residue was purified by silica gel (100-200 mesh) column chromatography by gradual elution from 5% to 10% EtOAc-petroleum ether. TLC system: 20% Ethyl acetate-petroleum ether, Rf value: 0.6 Nature of the compound: Colorless liquid, Yield: 3.5 g |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping