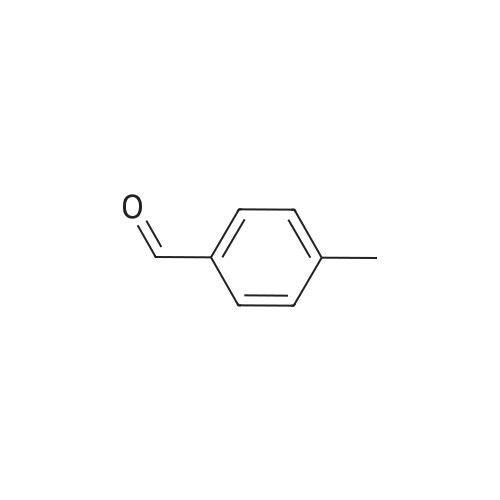
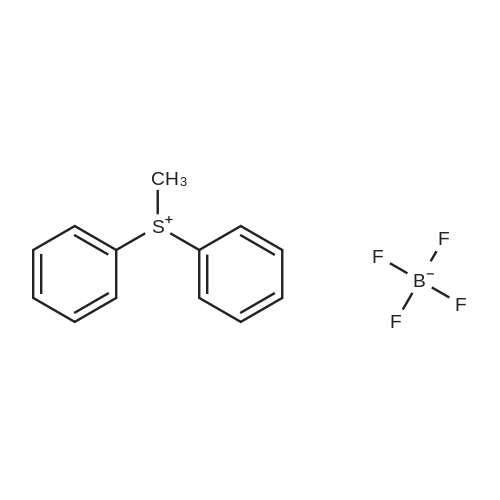
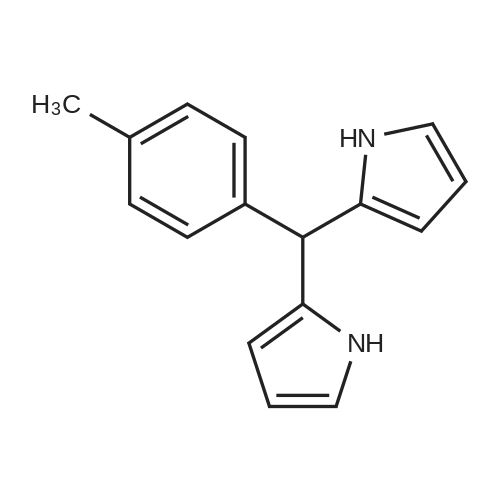
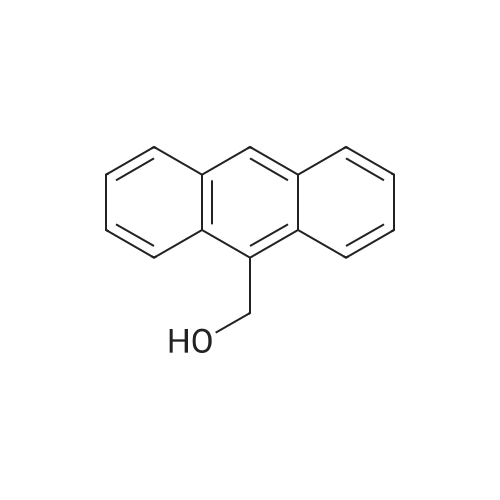
| 41.3% |
|
General procedure: (1) Prepare 560mol of pyrrole and 560mol of benzaldehyde into a mixed solution and set aside;(2) In a 2000L high-pressure titanium polymerization reactor 1 with a mechanical stirring and electric heating jacket and a distillation device on the top, add a solvent E consisting of 840L of propionic acid and 560L of cyclohexane,From the bottom of the polymerization reactor 1, N2 is bubbled to the oxygen concentration in the gas phase of the reactor top condenser outlet to be lower than 1%, and then the electrical heating jacket switch of the polymerization reactor 1 is started to heat the liquid in the polymerization reactor 1 to reflux. , Maintaining the pressure of the polymerization reactor 1 at 5.0atm, while adding the mixed solution prepared in step (1) from the top of the polymerization reactor 1 by dropwise addition, the molar concentration of pyrrole in the reaction solution is always kept lower than 0.1mol during the dropwise addition / L.The water produced by the reaction is removed from the system through a distillation device at the top of the polymerization reactor, and after condensing, it is delaminated by a liquid-liquid layering device 8 to circulate the upper oil phase back into the reaction system. The reflux ratio of the distillation column was continuously adjusted so that the water concentration in the polymerization reactor 1 was always lower than 0.05%. After the dropwise addition, the reaction was continued at the same time, and the reaction was stopped after 15 minutes. At the end of the reaction, the reaction temperature was 157.1 C. Then slowly cool it down. When the temperature in the polymerization reactor 1 drops to normal temperature, the reactants in the polymerization reactor 1 are filtered through the filtering and washing device 2 to obtain a filtrate and a crude TPP filter cake. The mass of the crude TPP filter cake is 54.8 kg. According to gas chromatography analysis, the amount of pyrrole remaining in the polymerization reaction system was 55.2 mol. The filtrate can be distilled into the polymerization solvent recovery tank 3 for distillation, and the solvent and unreacted pyrrole can be recovered. The recovered solvent and pyrrole are collected for recycling to the polymerization reactor 1 and continued to be used. The residue of the distillation kettle is discharged outside the system. deal with.(3) Add 54.8 kg of crude TPP filter cake obtained by centrifugal filtration to a 2000L oxidation reactor 4 with mechanical stirring and electric heating jacket, with condensation reflux at the top and gas distributor at the bottom, and simultaneously add 1400 kg of propylene into the kettle Acid, and simultaneously introducing nitrogen gas to replace the gas phase space in the oxidation reactor 4 to a tail oxygen concentration of less than 3%. Then, the switch of the electric heating jacket is started to heat the liquid in the oxidation reactor 4. When the liquid in the oxidation reactor 4 is heated to reflux, air is passed through to carry out the oxidation reaction. The oxidation reaction time is 22 minutes. During the reaction, the oxygen concentration in the tail gas is strictly controlled by adjusting the amount of the air to be passed to less than 3%. After the oxidation reaction was completed, it was left to cool slowly. When the temperature of the liquid in the oxidation reactor 4 is reduced to normal temperature, it is put into a filtering and washing device 5 for filtration to obtain a filtrate and a solid. The filtrate is dehydrated through an oxidation reaction solvent recovery tower 6 and dehydrated and recovered to obtain propionic acid, and the recovered propionic acid is collected. After that, it is ready to be used in the recycle and oxidation reactor 4 and the residue of the tower kettle is discharged out of the system. The filtrate can also be directly recycled to the oxidation reactor 4 without any treatment and continued to be used. The solid obtained by filtration was repeatedly washed with hot water and then filtered until the filtrate was colorless. Then repeatedly wash with methanol and then filter until the filtered wastewater is freecolor. The solid washed with methanol is the product TPP, and 46.0 kg of a tetraphenylporphine product is obtained after drying in a drying device 7 under vacuum at 80 C.The yield of the product TPP based on the pyrrole of the reactant was 59.3%, and the purity was 99.2%. |
| 23% |
|
In a round-bottom flask were placed dichloromethane (40 mL), p-tolualdehyde (470 μL, 4.0 mmol) and BF3O(Et)2 (68 μL, 0.26 mmol), followed by the dropwise addition of pyrrole (280 μL, 4.0 mmol). The resulting mixture was stirred for 30 min, at room temperature. Then, SeO2 (6.658 g, 60.0 mmol) was added. After 1 h stirring, the reaction was quenched by filtering the mixture through a pad of Celite. The filtrate was concentrated at reduced pressure. The resulting residue was purified by silica gel column chromatography, using dichloromethane/hexane (1:2). Porphyrin 10 was obtained at 23% yield. 1H-NMR (CDCl3) (ppm): 8.85 (s, 8H, pyrrole), 8.10 (m, 8H, o-Ph), 7.55 (m, 8H, m-Ph), 2.71 (s, 12H, CH3), -2.17 (s, 2H, NH). MALDI-TOF: m/z 671 [M+H]+ NMR spectral data is in accordance with the following reference: Mahmood, M. H.; Liu, H-Y; Wang, H.-H.; Jiang, Y.-Y.; Chang, C. K. Tetrahedron Lett., 2013, 54, 5853 - 5856. |
| 8% |
|
General procedure: Equimolar amounts of aldehyde and pyrrole (1:1) were mixed together in 150 mL dichloromethane. After totally dissolved, 0.5 mL trifluoroacetic acid (TFA) was added. The reaction mixture was refluxed for 3 days. Then the mixture was cooled down to room temperature and was quenched by adding triethylamine. 0.6 g of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) was added to the mixture and stirred for another 1 h. The purification procedure for 1 is given below: after the completion of reaction, the solvent was dried in vacuum and was purified by flash chromatography on neutral alumina using dichloromethane/triethylamine (V/V 20:1) eluent followed by basic alumina using dichloromethane. For analytical purpose, the nearly pure product was subjected to dichloromethane/ethyl acetate 1:1 mixture. The pure product was settled down in 20 minutes, filtered and washed with ethyl acetate. The compounds 2-5 were chromatographed on silica using dichloromethane as eluent. |
|
With hydrogenchloride; In methanol; water monomer; for 1h;Reflux; Inert atmosphere; |
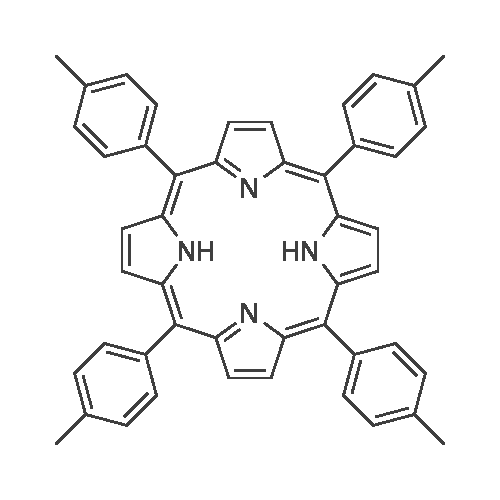
67 g (1 mol) of pyrrol was reflux-boiled with 92 g (1 mol) of 4-methylbenzaldehyde in 400 ml of methanol+20 ml conc. HCl in a nitrogen atmosphere for 1 hour, whereby a dark blue solution of tetratolyl porphyrine was spontaneously obtained. |
|
With propionic acid;Reflux; |
General procedure: Porphyrin ligands were synthesized by the method of Alder with some modifications [25]. In a 100 mL of flask with three necks,0.015 mol of corresponding benzaldehyde was dissolved in 30 mL of propionic acid solution. The mixture was heated at reflux temperature with vigorous stirring. Subsequently, 0.015 mol of freshly distilled pyrrole solved in propionic acid solution (5 mL) was slowly added intothe above mixture. After a period of time of reaction, the mixture solution was cooled to room temperature and placed in the refrigerator overnight. Then the purple solid was filtered and washed with hot water and ethanol and dried at 80 C for 8 h. The crude product was purified via column chromatography using neutral alumina (100-200 mesh size) with chloroform or dichloromethane as eluent. |
|
In propionic acid; at 145℃; for 2h;Inert atmosphere; Darkness; |
General procedure: In the typical procedureof porphyrin syntheses, benzaldehyde or its derivatives(150mmol) was heated to refluxing (145 C) with stirring in propionic acid (450mL) under the nitrogen protection,followed by the addition of redistilled pyrrole (150mmol)dropwise under refluxing conditions. After refluxing for2.0h in the shield from ambient light, the resultant mixturewas cooled in air to room temperature and kept standinguntil a large amount of solid appeared. Then the solid wascollected through suction filtration and suspended in methanol(200mL) with stirring for 6.0h. The collected purplishred precipitate was washed successively using methanol(2 × 100mL) and purified through silica column chromatographyseparation with cyclohexane and dichloromethaneas eluent (10:1-4:1, volume/volume). |
|
With propionic acid; at 145℃; for 2h;Inert atmosphere; Darkness; |
General procedure: Tetraphenylporphyrin (TPP), tetrakis(2-chlorophenyl)porphyrin (T(2-Cl)PP), tetrakis(3-chlorophenyl)porphyrin (T(3-Cl)PP), tetrakis(4-chlorophenyl)porphyrin (T(4-Cl)PP), tetrakis(4-fluorophenyl)porphyrin (T(4-F)PP), tetrakis(4-methylphenyl)porphyrin (T(4 CH3)PP), tetrakis(4-methoxyphenyl)porphyrin (T(4 OCH3)PP), tetrakis(naphthalen-2-yl)porphyrin (T(2-Na.)P) and tetrakis(4-methoxycarbonylphenyl)porphyrin (T(4 COOCH3)PP) were synthesizedthrough condensation of the corresponding aromatic aldehydewith freshly distilled pyrrole in propionic acid according to the Adler-Longo method after some modifications (Eq. 1 in Scheme 1) [74-76].In the synthesis process, aromatic aldehyde (100 mmol) was dissolved inpropionic acid (300 mL) with stirring at the room temperature under theatmosphere of nitrogen. Then, the resultant solution was heated torefluxing (145 C), and freshly distilled pyrrole (100 mmol) was addeddropwise. After stirring and refluxing for 2.0 h under the protection oftin foil from ambient light, the resultant reaction mixture was cooled toroom temperature and kept standing at room temperature for 24.0 h.The collected precipitate from suction filtration was suspended inmethanol (200 mL) with stirring at room temperature for 6.0 h andwashed with methanol (2 × 100 mL) successively. At last, silica columnchromatography was employed for further purification using themixture of cyclohexane and dichloromethane as eluent (Vcyclohexane :Vdichloromethane = 6 : 1). All the obtained porphyrins were dried at 75 C for12.0 h under reduced pressure and characterized through FT-IR, 1HNMR, 13C NMR and ESI-MS. Details could be seen in the ElectronicSupplementary Information. |
|
With propionic acid; at 141℃; for 0.5h; |
General procedure: Fig. 2a shows that the synthesis of H2TPP is performed via thereaction between propanoic acid (2L) and benzaldehyde (40 mL).Fig. 2b illustrates that H2TTPP is synthesized through the introductionof the 4-tolualdehyde (12.5 g) with the propanoic acid(110 mL). It is to be noted that the synthesis of these two adsorbentsare done through the application of the same procedurebased on the following steps: reflux for 30 min, then, allowing tocool, after that, filtration and finally, drying under-vacuum for3 h. The mass of the dried solid of H2TPP is equal to 9.76 g andthe one of the obtained solid H2TTPP is equal to 3 g. When dissolvedin chloroform, the solid compounds of porphyrins resultin two solutions having a concentration equal to 2.9 102 mol.L1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping