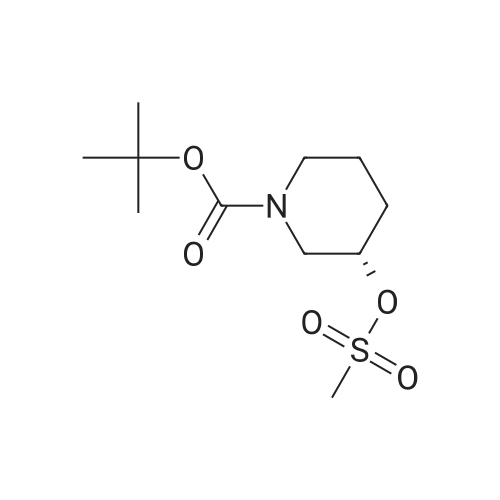
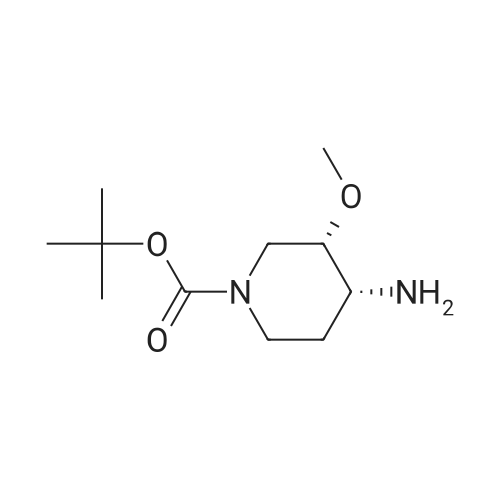
| 95% |
With triethylamine; In dichloromethane; at 0 - 20℃; for 1h; |
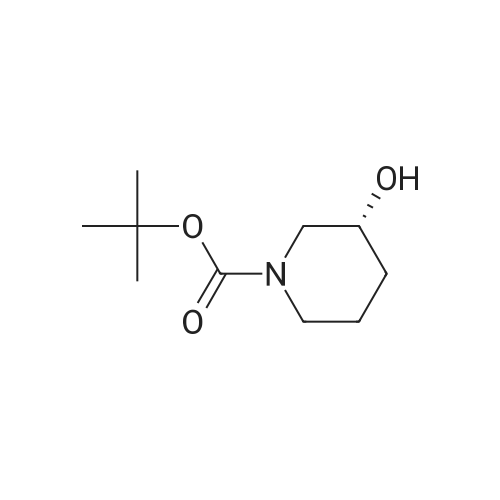
A 500 ml three-necked flask was charged with a solution of the compound prepared in any of Examples 5 (1) (2) (3)(S) -l-tert-butoxycarbonyl-3-hydroxypiperidine (30.28, 0.15 mol),Dichloromethane 180ml, triethylamine (18.28; 0.18mol), control 0 ~ 10 ° (3, dropping methyl sulfonyl chloride(18.9 g, 0.165 mol), stirred at room temperature for 1 hour,Water quenching, sub-aqueous phase, organic phase, washed, anhydrous sodium sulfateDried and concentrated to give 39.8 g of (S) -l-tert-butoxycarbonyl-3-methanesulfonylpiperidine. (Yield: 95percent; LC-MS: m / e = 279.1) (Theory: 41.9 g). |
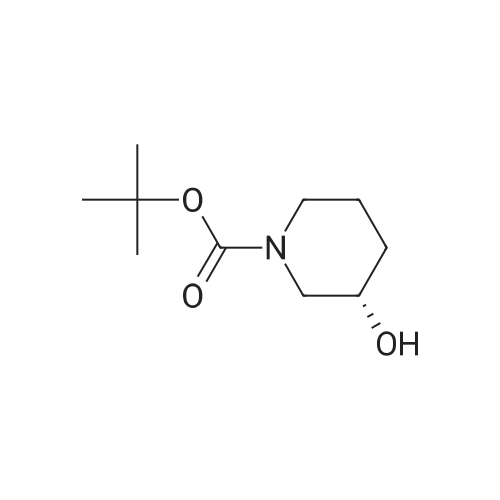
| 93% |
With triethylamine; In dichloromethane; at 0 - 20℃; for 2h; |
To a solution of (S)-tert-butyl 3-hydroxypiperidine-1-carboxylate (750 mg, 3.73 mmol) andTEA (1.88 g, 18.7 mmol) in DCM (10 mL) was added MsCI (550 mg, 4.85 mmol) at 0 °C.The solution was warmed to room temperature and stirred for 2 hrs. The mixture waswashed with H20 (10 mL x 2) and brine (10 mL x 2), dried over Na2SO4 and concentrated togive the desired product (970 mg, yield 93percent) as a yellow solid.1H NMR (300 MHz, CDCI3): 4.76-4.68 (m, 1H), 3.67-3.57 (m, 2H), 3.48-3.40 (m, 1H), 3.35-3.27 (m, 1H), 3.04 (s, 3H), 2.07-1.77 (m, 3H), 1.57-1.50 (m, 1H), 1.45 (s, 9H).LC-MS: N/A |
| 89% |
With triethylamine; In dichloromethane; at 0℃; for 2h; |
[0399] To a stirred solution of (S)-N-Boc-3-hydroxypiperidine (3 g, 17.1 mmol) and Et3N (2.62 ml, 18.8 mmol) in CH2Cl2 (85 ml) at 0° C methanesulfonyl chloride (1.33 ml, 17.1 <n="219"/>mmol) is added. After stirring at 0° C for 2 hours, the reaction is concentrated in vacuo and dissolved in ethyl acetate (100 ml). The reaction is washed with saturated NaHCpsi3 (50 ml) three times, brine, dried over MgSO4, filtered, and concentrated in vacuo to provide the title compound (4.25 g, 89percent). |
| 26.8 mg |
With triethylamine; In toluene; at 0℃; for 1h; |
20 g of (S)-N-Boc-3-pyridinol was dissolved in 100 ml of toluene, and 21 ml of triethylamine and 9.2 ml of methanesulfonyl chloride were added thereto at 0°C. The mixture was stirred for 1 hour under ice cooling, subsequently ethyl acetate and water were added thereto, and an organic layer was separated. The organic layer was washed with a saturated aqueous solution of sodium hydrogen carbonate, a saturated aqueous solution of ammonium chloride and water, and then was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and thus 26.8 g of the title compound was obtained as a colorless solid. |
| 31 g |
|
tert-Butyl (3S)-3-hydroxypiperidine-1-carboxylate (25 g) and triethylamine (30 g) were added to toluene (250 mL) at 25°C to obtain a reaction mixture. The reaction mixture was cooled to 0°C to 5°C. A solution of mesyl chloride (22.5 g) in toluene (100 mL) was added drop-wise to the solution over a period of one hour at 0°C to 5°C. Thereaction mixture was stirred for 2 hours at room temperature. Water (250 mL) was added to the reaction mixture, and the mixture was stirred to separate the organic layer. The organic layer was washed with water (100 mL), and then evaporated under vacuum to obtain a pale yellow viscous cmde material (52 g). Toluene (30 mL) was added at 45°C, and a solid was obtained by the slow addition of hexane (150 mL) over 15 minutes. Themixture was stirred for one hour at room temperature. The solid was filtered, and then kept in a vacuum oven at 45°C for 3 hours to obtain the title compound. |
|
With triethylamine; In dichloromethane; at 0℃; |
The (S)-3-hydroxy-piperidine-1-carboxylic acid t-butyl ester (4g, 19.88 mm, 1.0equiv) dissolved in dichloromethane (25 ml) in, cooling to 0 °C, add triethylamine (3.32 ml, 23 . 86 mm, 1 . 2equiv), slow adds by drops the armor sulfonyl chloride (1.69 ml, 21 . 86 mm, 1 . 1equiv) in dichloromethane (5 ml) solution, reaction is complete by TLC monitoring, adding water and methylene chloride, separating organic layer, respectively for 1N hydrochloric acid and physiological salt water washing, dry anhydrous sodium sulfate, filtered and concentrated, directly used for the next step reaction. |
| 5.4 g |
With dmap; triethylamine; In dichloromethane; at 0 - 30℃; for 2h; |
Methanesulfonyl chloride (3.7 gms) and 4-dimethylaminopyridine (0.66 gms) were added to a mixture of (S)-tert-butyl-3-hydroxypiperidine-1-carboxylate compound of formula-lO (5 gms) and dichioromethane (25 ml) at 0-5°C. Triethyl amine (7.5 gms) was slowly added to the reaction mixture at 0-5°C. Raised the temperature of the reaction mixtureto 25-30°C and stirred for 2 hrs. Water was added to the reaction mixture. Both the organic and aqueous layers were separated. The aqueous layer was extracted with dichioromethane. Organic layers were combined and cooled to 0-5°C and acidify the reaction mixture with 10percent HC1 solution. Both the organic and aqueous layers were separated. The organic layer was washed with aqueous sodium bicarbonate solution followed by aqueous sodium chloridesolution. Distilled off the solvent from the organic layer completely under reduced pressureand co-distilled with cyclohexane. Cyclohexane (150 ml) was added to the obtained compound at 25-30°C and stirred for 30 mins at the same temperature. Cooled the reaction mixture to 10-15°C and stirred for 60 mins at the same temperature. Filtered the precipitated solid, washed with cyclohexane and dried to get the title compound.Yield: 5.4 gms; Melting range: 85-90°C. |
| 31 g |
With triethylamine; In toluene; at 0 - 20℃; for 3h; |
Example 3: Preparation oftert-butyl (3S)-3-r(methylsulfonyl)oxylpiperidine-l-carboxylate (Formula IV. when Pr1 is tert-butoxycarbonyl (Boc) and Pr2 is mesyl) tert-Butyl (3S)-3-hydroxypiperidine-l-carboxylate (25 g) and triethylamine (30 g) were added to toluene (250 mL) at room temperature to obtain a reaction mixture. The reaction mixture was cooled to 0°C to 5°C. A solution of mesyl chloride (22.5 g) in toluene (100 mL) was added drop-wise over a period of 1 hour at 0°C to 5°C. The reaction mixture was stirred for 2 hours at room temperature. Water (250 mL) was added to the reaction mixture, and then the mixture was stirred to separate the organic layer. The organic layer was washed with water (100 mL), and then evaporated under vacuum to obtain a pale yellow viscous crude material (52 g). Toluene (30 mL) was added at 45°C, and a solid was obtained by the slow addition of hexane (150 mL) over a period of 15 minutes. The mixture was stirred for 1 hour at room temperature. The solid was filtered, and then kept in a vacuum oven at 45°C for 3 hours to obtain the title compound. Yield: 31 g |
| 26.8 g |
With triethylamine; In toluene; at 0℃; for 1h; |
(Step 1) Synthesis of (S)-tert-butyl 3-(methylsulfonyloxy)piperidine-1-carboxylate (0187) 20 g of (S)?N-Boc-3-piperidinol was dissolved in 100 mL of toluene, and 21 mL of triethylamine and 9.2 mL of methanesulfonyl chloride were added thereto at 0° C. The mixture was stirred for 1 hour under ice cooling, subsequently ethyl acetate and water were added thereto, and an organic layer was separated. The organic layer was washed with a saturated aqueous solution of sodium hydrogen carbonate, a saturated aqueous solution of ammonium chloride and water, and then was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and thus 26.8 g of the title compound was obtained as a colorless solid. |
| 26.8 g |
With triethylamine; In toluene; at 0℃; for 1h; |
(Step 1) Synthesis of (S)-tert-butyl 3-(methylsulfonyloxy)piperidine-1-carboxylate (S)-N-Boc-3-piperidinol (20 g) was dissolved in toluene (100 mL). To the solution, triethylamine (21 mL) and methanesulfonyl chloride (9.2 mL) were added at 0° C. The mixture was stirred under ice cooling for 1 hour, and then, ethyl acetate and water were added thereto to separate an organic layer. The organic layer was washed with a saturated aqueous solution of sodium bicarbonate, a saturated aqueous solution of ammonium chloride, and water and then dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 26.8 g of the title compound as a colorless solid. |
| 165.85 g |
With triethylamine; In dichloromethane; at -5 - 22℃; |
129.19g (0.6419 mol, leq.) crude tert-butyl (3S)-3-hydroxypiperidine-l-carboxylate was dissolved in 800ml dichloromethane. 142.88g (1.412 mol, 2.2 eq.) trimethylamine was added to the solution at room temperature (RT=21 -22°C). The reaction mixture was cooled to -5°C and 95.5g (0.834 mol, 1.3eq.) methansulionyl chloride was added dropwise to the reaction mixture. The ice bath was removed and the mixture stirred overnight at room temperature. 120 ml IN HCI solution were added to the reaction mixture to adjust the pH to 3-4. Two layers formed. The organic phase was isolated and the water phase extracted with 2x50ml dichloromethane. The organic phases were combined and the solvent evaporated. Light yellow crystals were obtained. The crystals were suspended in 700 ml water and stirred for 4h at room temperature. The crystals were filtered and dried under vacuum (yield 165.85g, 0.5938 mol, melting point 90-91°C). |
| 26.8 g |
With triethylamine; In toluene; at 0℃; for 1h; |
20 g of (S)?N-Boc-3-pyridinol was dissolved in 100 mL of toluene, and 21 mL of triethylamine and 9.2 mL of methanesulfonyl chloride were added thereto at 0° C. The mixture was stirred for 1 hour under ice cooling, subsequently ethyl acetate and water were added thereto, and an organic layer was separated. The organic layer was washed with a saturated aqueous solution of sodium hydrogen carbonate, a saturated aqueous solution of ammonium chloride and water, and then was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and thus 26.8 g of the title compound was obtained as a colorless solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping