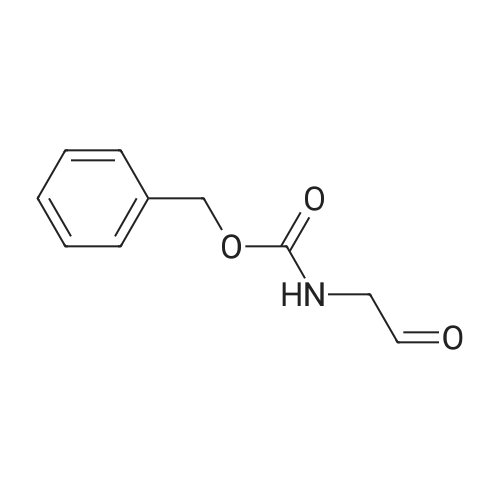
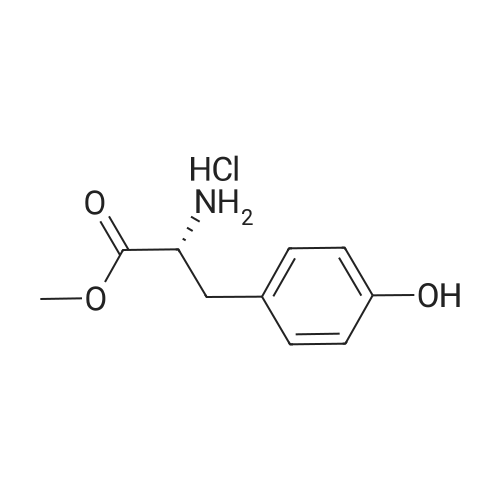
| 40% |
|
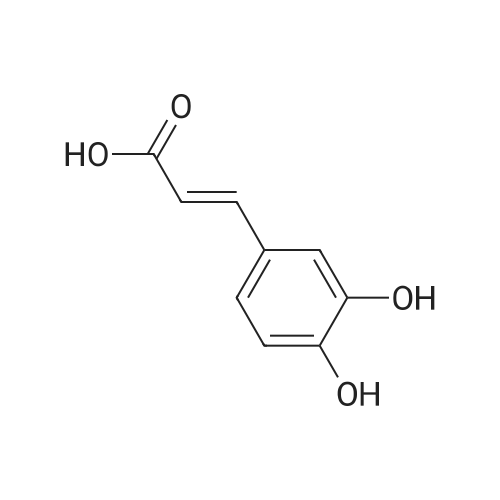
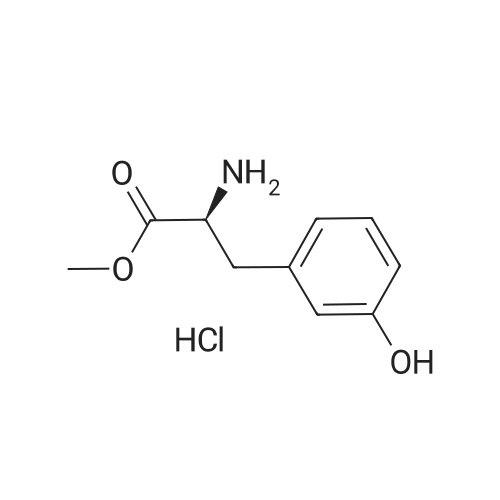
Caffeic acid (140.7 mg, 0.78 mmol) was dissolved in CH2Cl2/DMF (1:3, 3.0 mL), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide(EDC) (247 mL, 147 mmol) and 1-hydroxy-benzotriazole(HOBt) (75.4 mg, 0.56 mmol) were added to the solution. After themixture was cooled in an ice-water bath for 15 min, Et3N (156 mL,1.12 mmol) and compound a (138.3 mg, 0.56 mmol) were added tothe mixture. After stirring at room temperature for 19 h, the mixturewas poured into H2O (50 mL), extracted with EtOAc (50 mL 3), washed with 5% NaHCO3 (150 mL) and brine (150 mL), driedover MgSO4, filtered, and the solvent removed in vacuo. The residue was purified by silica gel column chromatography (CHCl3:MeOH =9:1) to afford clovamide methyl ester (clovamide-Me, 10, 84.7 mg,40%) as a yellow powder: 1H NMR (CD3OD) d 7.36 (1H, d, J = 15.7Hz, H-70), 6.99 (1H, d, J = 1.9 Hz, H-2), 6.89 (1H, dd, J = 8.2, 1.9 Hz,H-6), 6.75 (1H, d, J = 8.2 Hz, H-5), 6.64 (1H, d, J = 1.9 Hz, H-20),6.67 (1H, d, J = 8.1 Hz, H-50), 6.53 (1H, dd, J = 8.1, 1.9 Hz, H-60),6.41 (1H, d, J = 15.7 Hz, H-80), 4.69 (1H, m, H-8), 3.68 (3H, s,MeO-90), 3.02 (1H, dd, J = 13.9, 5.9 Hz, H-7a), 2.93 (1H, dd, J =13.9, 6.6 Hz, H-7b); 13C NMR (CD3OD) d 174.6 (C, C-9), 169.8(C, C-90), 149.5 (C, C-40), 147.3 (C, C-30), 146.8 (C, C-3), 145.9(C, C-4), 143.8 (CH, C-70), 130.2 (C, C-1), 128.9 (C, C-10), 123.1(CH, C-60), 122.4 (CH, C-6), 118.4 (CH, C-80), 118.0 (CH, C-2),117.2 (CH, C-5), 117.1 (CH, C-50), 115.9 (CH, C-20), 56.5 (CH, C-8),53.4 (CH3, CO-9) 39.1 (C, C-7); UV kmax (MeOH) nm (e): 208(12400), 220 (12100), 291 (9700), 324 (10700); HR-ESI-MS (negativeion) m/z: 372.1100 [MH] (calcd for C19H18NO7, 372.1083);[a]D20 +20 (c = 1.0, MeOH). |
|
With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In acetonitrile; at 20℃; |
General procedure: As shown in Scheme 1, the synthetic route of the analogues (1-7) involved a two-step sequence viamethyl esterification of L-amino acidand amide condensation. 100 muL SOCl2was added in portions to 4 mL methanol at -10 C,then 1 mmol L-amino acid was addedand the mixture was warmed to room temperature and stirred overnight. After thesolvent was removed, 5 mL CH3CN, 500 muL DIPEA (N,N-Diisopropyl ethylamine), 1.1 mmolcorresponding substituted acid and 1.1 mmol HBTU (O-Benzotriazole-N,N,N',N'-tetramethyl-uronium-hexafluorophosphate)was added into the residue. The mixture was stirred for 1 h at room temperatureto finish condensation. The reaction solution was added 20 mL 1 M HCl, andextracted with ethyl acetate (4 × 20 mL). The combined organic phasewas dried over anhydrous Na2SO4 and finally evaporated invacuum. The residue was purified by silica-gel chromatography using mixtures ofPE/EtOAcas eluent to afford compounds 1-7.At this stage, all compounds were fully analyzed and characterized by nuclearmagnetic resonance (NMR), high resolution massspectrum (HRMS). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping