|
|
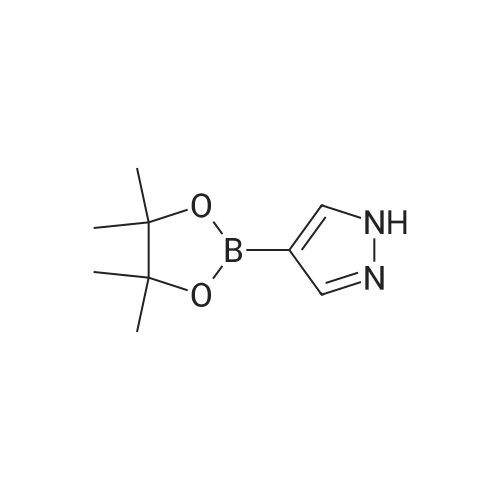
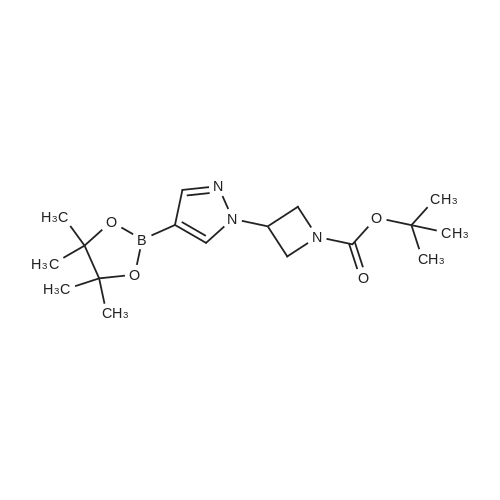
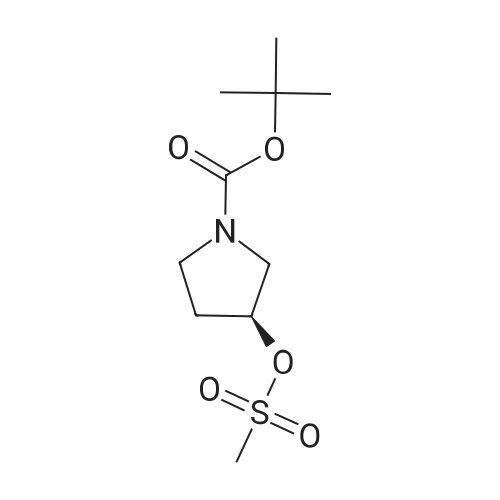
NaH (60% in mineral oil, 222 mg, 5.6 mmol) is added portionwise to a stirred solution of A- (4,4,5,5-Tetramethyl-[1 ,3,2]dioxaborolan-2-yl)-1H-pyrazole (1.10 g, 5.56 mmol) in DMF (20 ml). The resulting mixture is stirred for 1 h at O0C and then allowed to warn to RT. A solution of 3-Methanesulfonyloxy-azetidine-i-carboxylic acid tert-butyl ester (as obtained in <n="61"/>preparation 80, 1.39 g, 5.56 mmol) in DMF (3 ml) is then added dropwise. After complete addition, the reaction mixture is heated at 95C for 5h. The reaction is quenched with water and extracted with EtOAc several times. The combined organic layers are washed with brine, dried over Na2SO4, filtered and the filtrate is concentrated in vacuo. The residue is purified by chromatography on a 40 g silica gel column on a Combiflash Companion (Isco Inc.) apparatus ( gradient CH2CI2: TBDME from 1 :0 => 0:1 ) to afford the title compound as a colorless foam, R1 = 1.200 min (Acquity UPLC BEH C18, 2.1x50mm, 1.7 micron, detection 215nM, 0.1 min 2% CH3CN in H2O , 2% to 100% CH3CN in H2O in 1.5min, 0.4 min 100% CH3CN + 0.1% TFA, flow rate 1.Oml/min); MS: 350 (M+1)+ . |
|
|
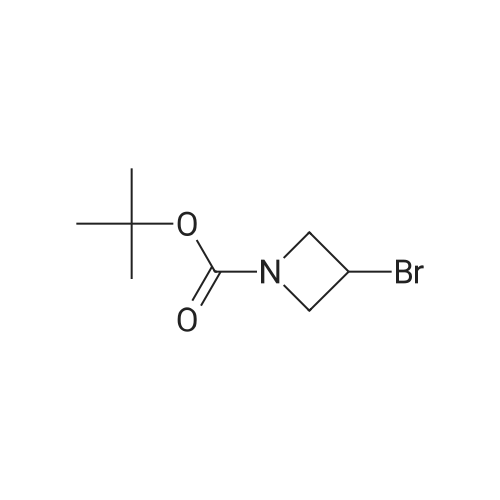
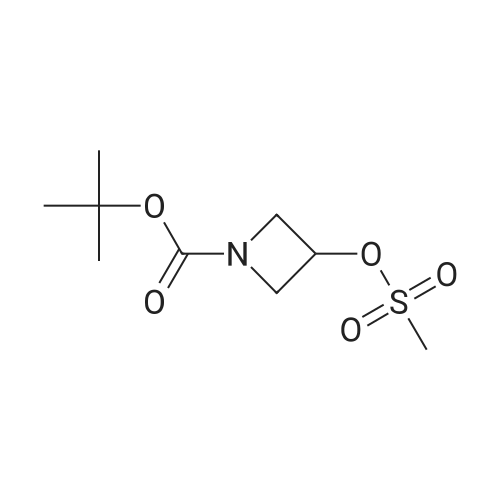
Stage 146.3 <n="119"/>; 3-[4-(4,4,5,5-Tetramethyl-[1 ,3,2]dioxaborolan-2-yl)-pyrazol-1-yl]-azetidine-1-carboxylic acid tert-butyl ester; The title compound was prepared as described in published patent application WO 2008148867: To a solution of 1-BOC-3-hydroxyazetidine (1.0 g, 5.77 mmol), 4-dimethylaminopyridine (7 mg, 0.058 mmol) and triethylamine (0.880 ml_, 6.35 mmol) in DCM (15 mL) cooled at 0 0C was added dropwise methanesulfonyl chloride (0.45 mL, 5.77 mmol). The RM was stirred 18 h at rt, diluted with DCM, washed with a solution of saturated NaHCO3 and brine. The organic layer was dried over Na2SO4, filtered and the solvent was removed under reduced pressure. 3-Methanesulfonyloxy-azetidine-i-carboxylic acid tert-butyl ester obtained as an oil was used directly in the next step.To a solution of 4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)-1H-pyrazole (1.4 g 5.57 mmol) in DMF (20 mL) stirred at 0 0C was added sodium hydride (55 % in oil, 0.243 g, 5.57 mmol). After addition the mixture was stirred 1 h at rt, a solution of the above obtained 3- methanesulfonyloxy-azetidine-1-carboxylic acid tert-butyl ester (1.4 g, 5.57 mmol) in DMF (3 mL) was added. The RM was stirred 30 min at rt, and then 3 h at 95 0C. After cooling at rt the mixture was poured in ice water and extracted with EtOAc. The combined organic phases were washed with a solution of saturated NaHCO3 and brine. The organic layer was dried over Na2SO4, filtered and the solvent was removed under reduced pressure. The residue was purified by flash chromatography (with heptane and EtOAc as eluants) to afford the title compound as an oil (tR 4.33 min (conditions 3), MH+ = 350.1) |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 100℃; for 14h;Inert atmosphere; |
A solution of 4-(4.4.5.5-tctramcthyl- 1.3.2-dioxaborolan-2-yl)- 1H-pyrazolc (1.24 g, 6.37 mmol), tert- butyl 3-[(methanesulfonyl)oxy]azetidine-l-carboxylate (1.60 g, 6.37 mmol) and caesium carbonate (3.32 g, 10.2 mmol) in N.N-d i m c th y 1 fo rm am i dc (15 ml) was stirred at 100 C under argon for 14 h. The reaction mixture is mixed with water and filtered. The filtrate was purified directly by HPLC to yield 513 mg (87 % purity, 20 % yield) of the title compound.LC-MS (method 2): Rt= 1.12 min; MS (ESIpos): m/z = 350 [M+H]+-NMR (600 MHz, DMSO-d6) d [ppm]: 1.25 (s, 12H), 1.40 (s, 9H), 4.06 - 4.15 (m, 2H), 4.22 - 4.31 (m, 2H), 5.19 - 5.25 (m, 1H), 7.71 (s, 1H), 8.07 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping