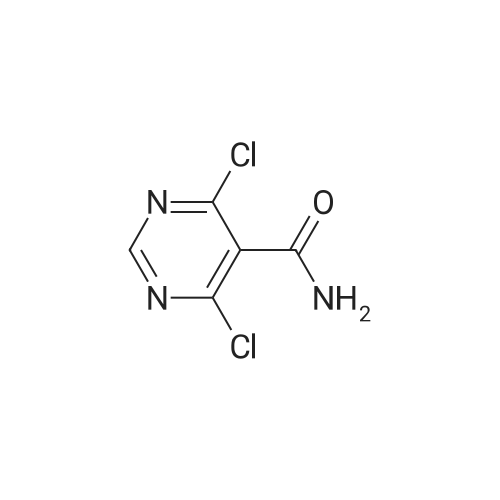
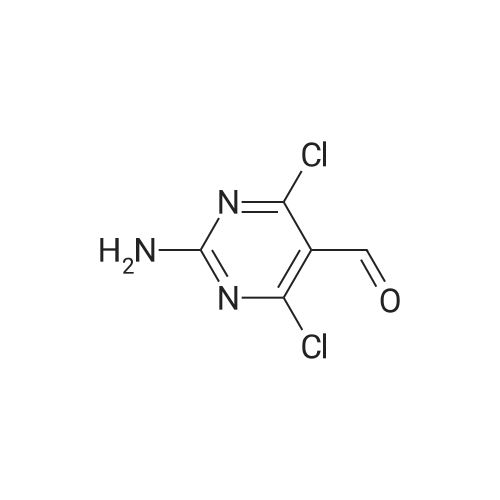
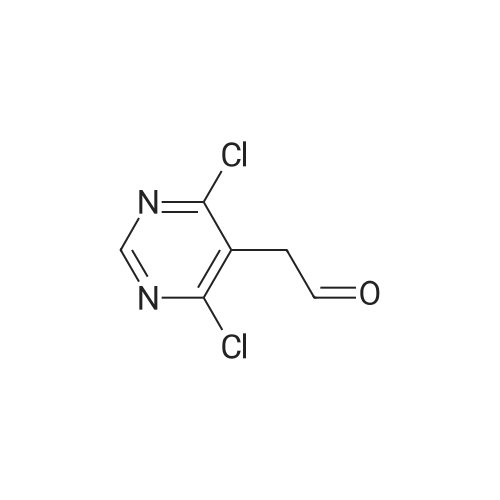
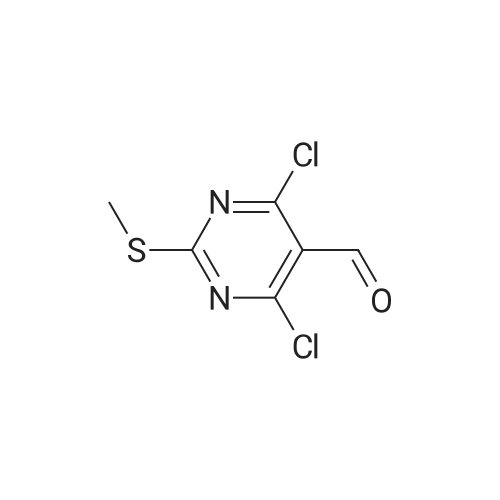
| 59% |
With trichlorophosphate; at 0℃;Reflux; |
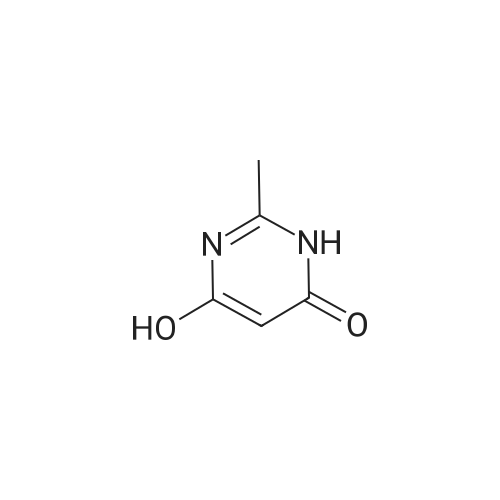
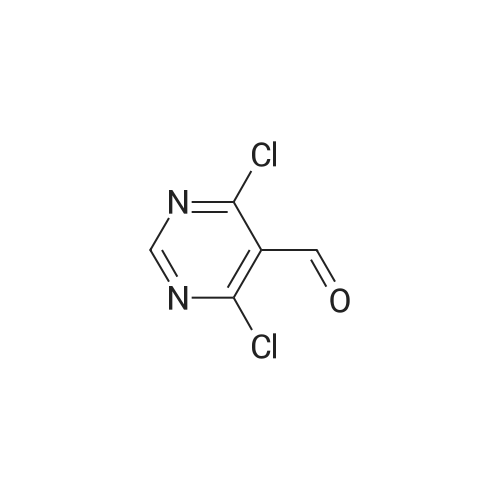
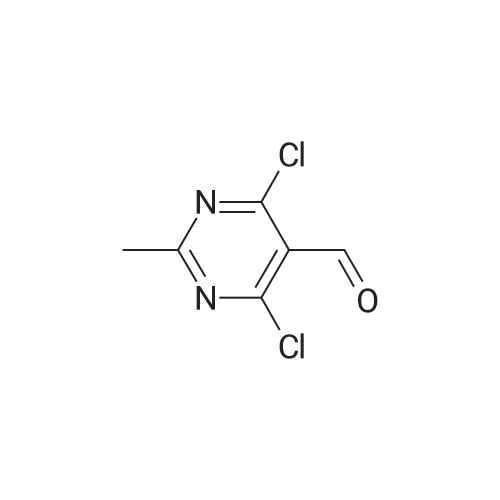
To POCl3 (60.6g, 397mmol) was added dropwise DMF (9.8g, 135mmol) at 0C. The resulting suspension was stirred at the same temperature for 1h. Then <strong>[1194-22-5]2-methylpyrimidine-4,6-diol</strong> 64 (10g, 79mmol) was added in portions and stirred at room temperature for 1h, followed by stirring at reflux overnight. The reaction solution was concentrated and diluted with cold ethyl acetate (100mL). This solution was added dropwise into ice-water and filtered. The filtrate was extracted with ethyl acetate (200mL). The organic layer was dried over Na2SO4, filtered and evaporated to give the desired product (9g, 59%) as a yellow oil. 1H NMR (400MHz, CDCl3) delta 10.41 (s, 1H), 2.75 (s, 3H). 13C NMR (150MHz, CDCl3) delta 185.7, 171.6, 162.6, 121.9, 26.1. MS (ESI/APCI) m/z 222.8 [M+MeOH+H]+. |
| 45% |
With trichlorophosphate; at 10 - 120℃; for 11h; |
Reference Example 1 Production of 4,6-dichloro-2-methylpyrimidine-5-carbaldehyde To ice-cooled phosphorus oxychloride (52.2 g, 340 mmol) was added dropwise DMF (3.10 mL, 40 mmol), and the mixture was stirred at room temperature for 30 min. 4,6-Dihydroxy-2-methylpyrimidine (5.04 g, 40 mmol) was added by small portions, and the mixture was stirred at room temperature for 1 hr and then at 120C for 10 hr. After cooling, the reaction mixture was poured into ice. Ethyl acetate and diethyl ether were added thereto, and the insoluble material was filtered off, and the filtrate was extracted 3 times with ethyl acetate/diethyl ether=1/4 solution. The extract was washed successively with water and brine, dried over magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluate, hexane:ethyl acetate=95:5?75:25) to give the title compound (3.46 g, 45%) as a yellow solid. 1H NMR (300 MHz, CDCl3) delta:2.78 (3 H, s), 10.44 (1 H, s). |
| 10 g |
|
To POCl3(60.6 g, 396.5 mmol) was added dropwise DMF (9.8 g, 134.8 mmol) at 0C. The resulting suspension was stirred at the same temperature for lh. Then <strong>[1194-22-5]2-methylpyrimidine-4,6-diol</strong> (10 g, 79.3 mmol) was added in portions and stirred at room temperature for lh, after that, stirred at 105 C overnight. The reaction solution was concentrated and diluted with cold ethyl acetate (lOOmL). This solution was added dropwise into ice-water, filtered and the filtrate was extracted with ethyl acetate (200 mL). The combined organic layer was dried over Na2S04, filtered and evaporated to give a yellow oil (10 g). This oil was dissolved in THF (50 mL) and water (10 mL), and NaBH4(4 g, 104.7 mmol) was added in portions at 0C. The resulting suspension was stirred at the same temperature for 30min. Water (50 mL) was added, and extracted with ethyl acetate (50 mL). The organic layer was dried over Na2S04, filtered and evaporated. The residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 10/1) to give the desired product (2 g, 20%) as a yellow solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping