| 75% |
With triethylamine; In methanol; at 0 - 50℃; for 20.5h; |
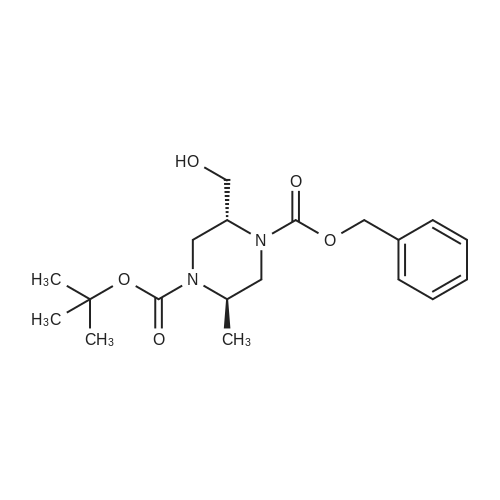
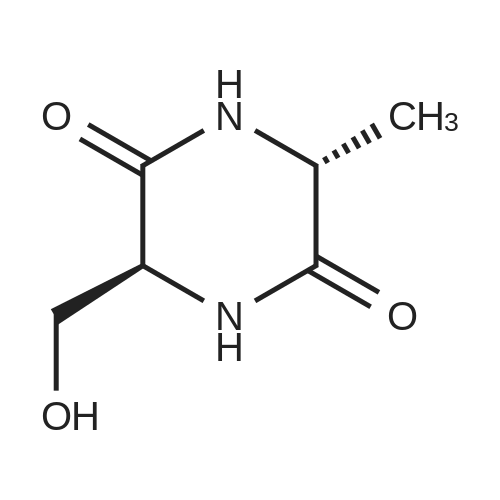
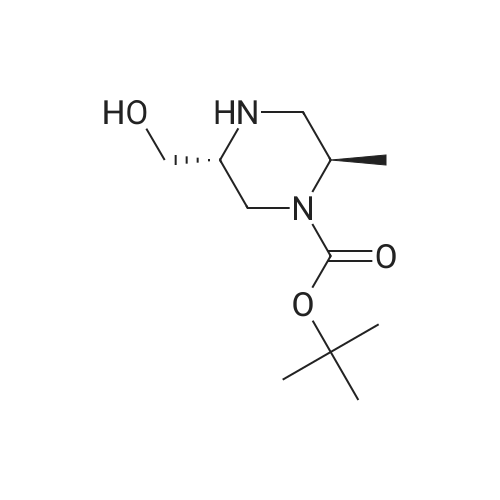
To ((2R,5R)-5-methyl-piperazin-2-yl)-methanol hydrochloride (20 g, 119 mmol) in MeOH (96 mL) at 0 C (ice bath) was added triethylamine (48.7 mL, 357 mmol). teit-Butyl dicarbonate (61 g, 280 mmol) in MeOH (145 mL) was added over 30 mm. The reaction temperature was maintained at <10 c for 1 h, warmed to ambient temperature over 1 h and then heated to 50 00 for 18 h. The reaction was concentrated and the residue dissolved in ethanol (397 mL). Asolution of NaOH (23.8 g, 595 mmol) in water (397 mL) was added and the reaction heated to 100 00 for 18 h, then cooled to ambient temperature. Mixture was neutralised with 1M HCI (-300 mL) to pH 9 (using a pH meter), then extracted with chloroform (3 x 700 mL), dried over sodium sulfate, filtered and concentrated. The residue was redissolved in MeOH and concentrated, then dried in vacuo at 40 c to give the title compound (21 g, 75%) as acolourless solid. 1H NMR (Me-d3-OD): 4.20-4.07 (1H, m), 3.79 (1H, dd), 3.71-3.58 (2H, m),3.54 (1H, dd), 3.24 (1H, dd), 3.18-3.01 (1H, m), 3.01-2.89 (1H, m), 2.55 (1H, dd), 1.48 (9H,5), 1.25 (3H, 5). |
| 75% |
|
Preparation 4: (2R,5R)-5-Hydroxymethyl-2-methyl-pi perazi ne-I -carboxylic acid tert-butyl esterTo ((2R,5R)-5-methyl-piperazin-2-yl)-methanol hydrochloride (20 g, 119 mmol) in MeOH (96mL) at 0 C (ice bath) was added triethylamine (48.7 mL, 357 mmol). teit-Butyl dicarbonate (61g, 280 mmol) in MeOH (145 mL) was added over 30 mm. The reaction temperature wasmaintained at <10 c for 1 h, warmed to ambient temperature over 1 h and then heated to 50 cfor 18 h. The reaction was concentrated and the residue dissolved in ethanol (397 mL). Asolution of NaOH (23.8 g, 595 mmol) in water (397 mL) was added and the reaction heated to100 00 for 18 h, then cooled to ambient temperature. Mixture was neutralised with 1M HCI(-300 mL) to pH 9 (using a pH meter), then extracted with chloroform (3 x 700 mL), dried over sodium sulfate, filtered and concentrated. The residue was redissolved in MeOH andconcentrated, then dried in vacuo at 40 c to give the title compound (21 g, 75%) as acolourless solid. 1H NMR (Me-d3-OD): 4.20-4.07 (1H, m), 3.79 (1H, dd), 3.71-3.58 (2H, m), 3.54 (1H, dd), 3.24 (1H, dd), 3.18-3.01 (1H, m), 3.01-2.89 (1H, m), 2.55 (1H, dd), 1.48 (9H, 5), 1.25 (3H, 5). |
| 75% |
|
To ((2 R, 5R)-5-methyl-pi perazin-2-yl)-methanol hydrochloride (which may be prepared asdescribed in Preparation 3) (20 g, 119 mmol) in MeOH (96 mL) at 0C (ice bath) was added triethylamine (48.7 mL, 357 mmol). teit-Butyl dicarbonate (61 g, 280 mmol) in MeOH (145 mL) was added over 30 mm. The reaction temperature was maintained at <10 c for 1 h, warmed to ambient temperature over 1 h and then heated to 50 c for 18 h. The reaction was concentrated and the residue dissolved in ethanol (397 mL). A solution of NaOH (23.8 g, 595 mmol) in water(397 mL) was added and the reaction heated to 100 c for 18 h, then cooled to ambient temperature. Mixture was neutralised with 1 M HCI (-300 mL) to pH 9 (using a pH meter), then extracted with chloroform (3 x 700 mL), dried over sodium sulfate, filtered and concentrated. The residue was redissolved in MeOH and concentrated, then dried in vacuo at 40 C to give the title compound (21 g, 75%) as a colourless solid. 1H NMR (Me-d3-OD): 4.20-4.07 (1H, m),3.79 (1H, dd), 3.71-3.58 (2H, m), 3.54 (1H, dd), 3.24 (1H, dd), 3.18-3.01 (1H, m), 3.01-2.89 (1H, m), 2.55 (1H, dd), 1.48 (9H, 5), 1.25 (3H, 5). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping