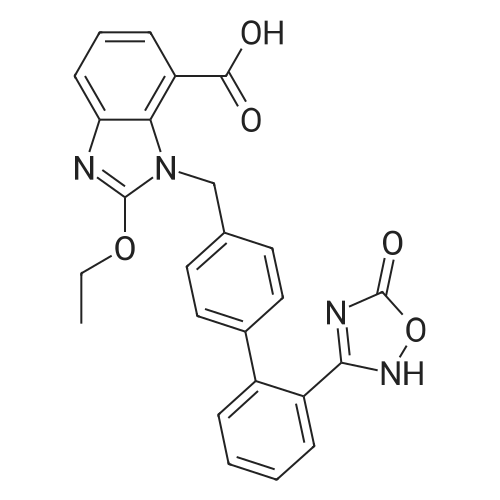
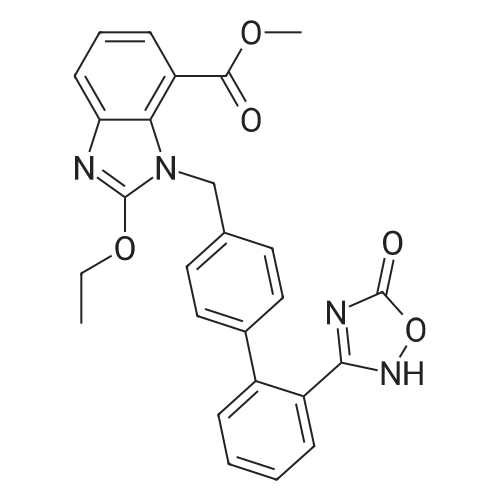
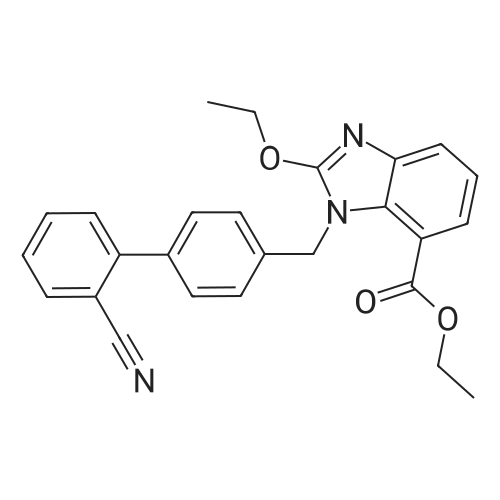
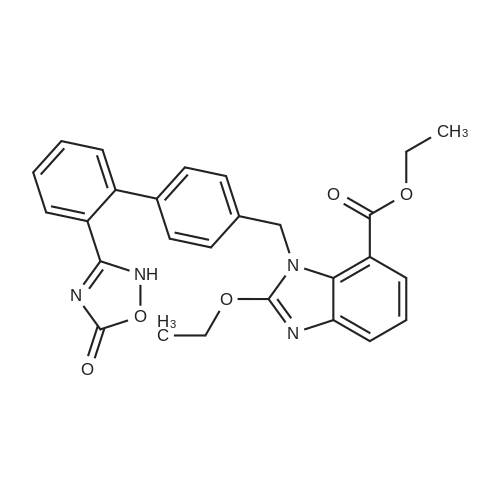
| 86% |
With acetic acid; at 80℃; for 1h; |
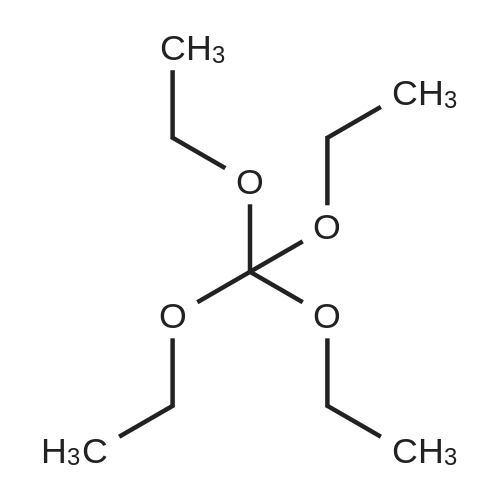
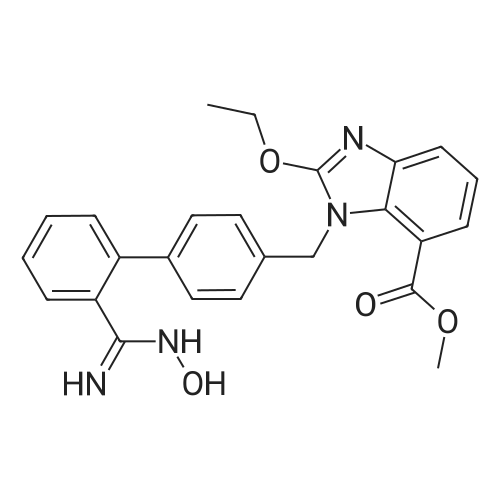
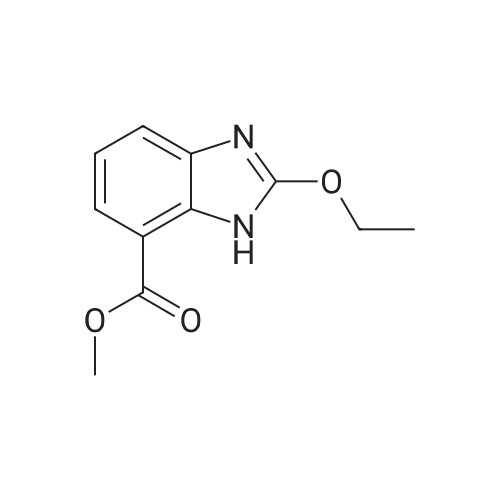
To a solution of compound 8 in tetraethyl orthocarbonate (5 mL), acetic acid (0.37 g) was added, and the mixture was stirred at 80 °C for 1 h, the reaction mixture was concentrated, and the residue was dissolved in ethyl acetate. Washed with water, evaporated to dryness, The crystal was recrystallized from ethyl acetate-hexane.Obtained 2.01g of colorless crystal,Yield 86percent, compound 5. |
| 84.8% |
With acetic acid; at 78 - 82℃; for 1 - 2h;Heating / reflux; Industry scale; |
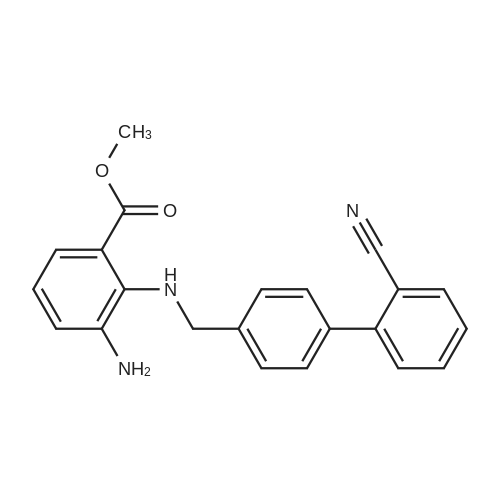
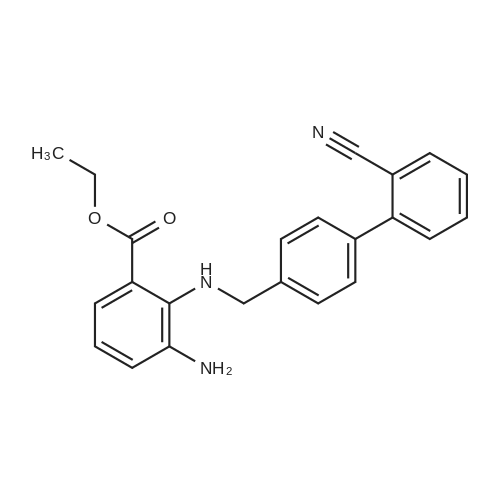
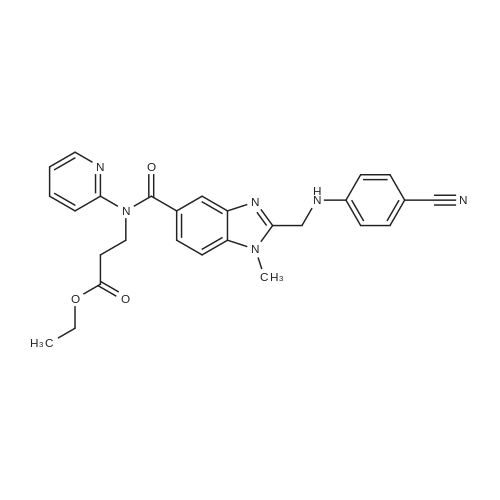
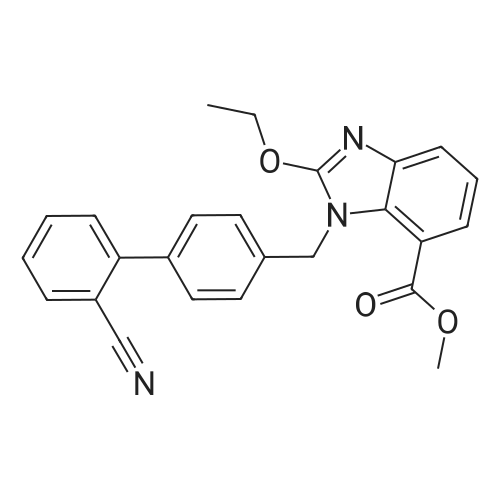
The concentrate of methyl 3-amino-2-N-[(2'-cyanobiphenyl-4-yl)methyl]aminobenzoate [MBA] obtained in Reference Example5, tetraethyl orthocarbonate [TEC] obtained in Reference Example 6 (397 kg) and acetic acid (62 kg) were mixed, and the mixture was heated (78 to 82°C) under reflux for about 1 to 2 hrs.. The reaction solution was cooled, and methanol (1680 L), a 24percent aqueous sodium hydroxide solution (65 L) and water (2030 L) were added.. The mixture was stirred at 60 to 30°C for 2 hours, and the PH was adjusted to 5 to 7.. After cooled to 5°C or lower, the precipitated crystals were separated, and washed with cold water (2500 L) and cold ethyl acetate (500 L) to give first crystals.. The mother liquor and the washing were concentrated under reduced pressure, followed by cooling to 5°C or lower, and the precipitated crystals were separated, and washed with cold ethyl acetate (20 L) to give second crystals.. The first and second crystals were combined, and dissolved in ethyl acetate (4890 L) under reflux.. A seed crystal was added at about 70°C, and cooled to 5 °C. The crystals were separated, and washed with cold ethyl acetate (200 L), followed by drying to give methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxybenzimidazole-7-carboxylate [BEC](361 kg, 84.8percent). mp. 168.5-169.5°C 1H-NMR(200MHz, CDCl3) delta: 1.42(3H,t), 3.71(3H,s), 4.63(2H,q), 5.59(2H,s), 7.09(2H,d), 7.20(1H,t), 7.45-7.59(5H,m), 7.69-7.80(2H,m), 7.92(1H,dd) IR(KBr) cm-1: 2225, 1725, 1550, 1480, 1430, 1280, 1250, 1040, 760, 750 |
|
With acetic acid; In toluene; at 20℃;Reflux; |
Example 1; To the solution of Methyl-3-amino-2-[[2'-cyanobiphenyl-4-yl]methyl]amino] benzoate (100 gm) in toluene (500 ml), tetraethyl ortho carbonate (125 gm) and acetic acid (20 gm) were added at room temperature and refluxed for 6 hours.Distilled off toluene under vacuum below 600C, added methanol (300 ml) at 550C, stirred for 20 minutes, cooled to room temperature, filtered and then washed with methanol (90 ml). Dried for 6 hours to yield Methyl-1-[(2-cyano biphenyl-4- yl)methyl]-2-ethoxy benzimidazole-7-carboxylate (90 gm, HPLC purity: 98.9percent). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping