Alternatived Products of [ 139163-43-2 ]
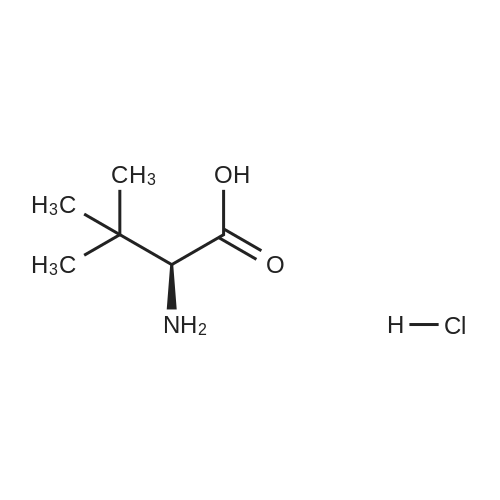
Product Details of [ 139163-43-2 ]
| CAS No. : | 139163-43-2 |
MDL No. : | MFCD07368368 |
| Formula : |
C6H14ClNO2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | OLMBOHVAVKHHTK-PGMHMLKASA-N |
| M.W : |
167.63
|
Pubchem ID : | 12314685 |
| Synonyms : |
|
Chemical Name : | (S)-2-Amino-3,3-dimethylbutanoic acid hydrochloride |
Safety of [ 139163-43-2 ]
Application In Synthesis of [ 139163-43-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 139163-43-2 ]
- 1
-
 [ 175416-53-2 ]
[ 175416-53-2 ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
- 2
-
C13H19NO2*HCl
[ No CAS ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
- 3
-
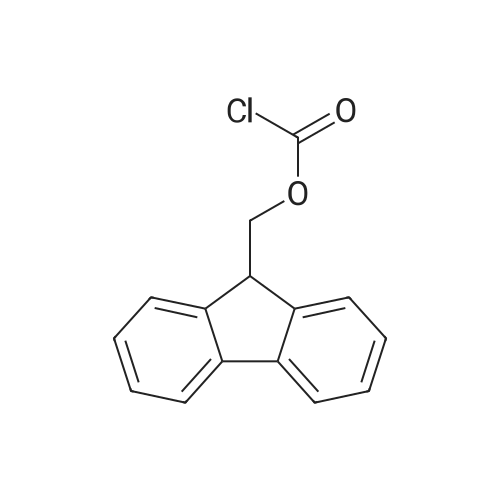 [ 28920-43-6 ]
[ 28920-43-6 ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]

-
 [ 132684-60-7 ]
[ 132684-60-7 ]
- 4
-
 [ 929220-40-6 ]
[ 929220-40-6 ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
- 5
-
 [ 929220-45-1 ]
[ 929220-45-1 ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
- 6
-
 [ 1775-74-2 ]
[ 1775-74-2 ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
- 7
-
(4S)-2-(2,2-dimethylpropyl)-4-phenyl-4,5-dihydrooxazole
[ No CAS ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
- 8
-
 [ 175226-69-4 ]
[ 175226-69-4 ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
- 9
-
 [ 62965-57-5 ]
[ 62965-57-5 ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
With hydrogenchloride; water; for 24.0h;Heating / reflux; |
Synthesis of (S)-2-amino-3,3-d9-dimethylbutanoic acid hydrochloride (XXV, R2/3 = C(CD3)3).; A mixture of compound XXIIc (R2 = R3 = C(CD3)3) (31.0 g, 222.6 mmol) in 6M aqueous HCI solution (1.5 L) was heated at reflux for 24 hrs. The mixture was concentrated in vacuo to give the crude product. The solid was redissolved in water (500 mL) and washed with EtOAc (2x200mL) to remove impurities from previous steps. The aqueous layer was then concentrated in vacuo, chased with toluene, and dried under vacuum at 50C to afford the HCl salt of the desired compound (S)-2-amino-3,3-dimethylbutanoic acid-d9 hydrochloride (XXV, R2 = R3 = C(CD3)3) (33.6 g, 85% yield) as a white solid, |
- 10
-
 [ 43049-56-5 ]
[ 43049-56-5 ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]

-
 [ 1092539-96-2 ]
[ 1092539-96-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
Synthesis of (S)-2-(d3-methoxycarbonylamino)-3,3-d9-dimethylbutanoic acid (XVII-d12).; To a solution of compound XXV (R2 = R3 = C(CD3)3) (4.42 g, 25.0 mmol) in a mixture of dioxane (12.5 mL) and 2M NaOH solution (60 mL) was added methyl chloroformate-d3 (5.0 g, 50.0 mmol, Cambridge Isotopes, 99 atom% D) dropwise, keeping the internal temperature below 50C. The resulting mixture was warmed to 60 C and stirred overnight, and then cooled to It The mixture was washed with dichloromethane and the aqueous layer was acidified with cone. HCl to pH = 2 and extracted with EtOAc. The combined extracts were dried, filtered, and concentrated in vacuo to afford the desired compound (S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid-d12 (XVII-d12) (3.8 g) as a yellow oil. |
- 11
-
L-tert-leucine dibenzoyl-d-tartrate salt
[ No CAS ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrogenchloride; In water; for 12.0h; |
Example 2 A mixture of L-tert-leucine.dibenzoyl-d-tartrate salt (27 g) as obtained in example-1, water (150 mL) and concentrated hydrochloric acid (50 mL) was stirred for 12 hours. The liberated dibenzoyl-d-tartaric acid was filtered and dried (20 g). The filtrate was concentrated under reduced pressure to remove all the solvent. The residue obtained was stirred with acetone (15 ml*2), filtered and dried to obtain colorless solid of L-tert-leucine hydrochloride salt. |
- 12
-
 [ 33105-81-6 ]
[ 33105-81-6 ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
- 13
-
 [ 139163-43-2 ]
[ 139163-43-2 ]

-
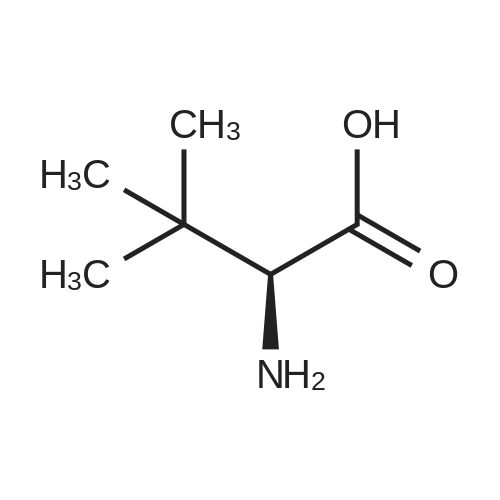 [ 20859-02-3 ]
[ 20859-02-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With epichlorohydrin; In toluene; |
It was suspended in toluene (50 mL) and epichlorohydrin (5.8 g) was added. The reaction mixture was stirred till the pH was neutral. It was filtered, the solid obtained was stirred with acetone (15 ml*2) and again filtered to obtain L-tert-leucine (5.6 g, 75% yield, 98.5% chemical purity, 99.9% chiral purity). |
|
With epichlorohydrin; In toluene; |
A mixture of L-tert-leucine.dibenzoyl-d-tartrate salt (27 g) as obtained in Example-1, water (150 mL) and concentrated hydrochloric acid (50 mL) was stirred for 12 hours. The liberated dibenzoyl-d-tartaric acid was filtered and dried (20 g). The filtrate was concentrated under reduced pressure to remove all the solvent. The residue obtained was stirred with acetone (15 ml x 2), filtered and dried to obtain colorless solid <strong>[139163-43-2]L-tert-leucine hydrochloride</strong> salt. It was suspended in toluene (50 mL) and epichlorohydrin (5.8 g) was added. The reaction mixture was stirred until the pH was neutral. It was filtered, the solid obtained was stirred with acetone (15 ml x 2) and again filtered to obtain L-tert-leucine (5.6 g, 75 % yield, 98.5 % chemical purity, 99.9% chiral purity). |
- 14
-
L-tert-leucine dibenzoyl-D-tartrate
[ No CAS ]

-
 [ 139163-43-2 ]
[ 139163-43-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrogenchloride; In water; for 12.0h; |
A mixture of L-tert-leucine.dibenzoyl-d-tartrate salt (27 g) as obtained in Example-1, water (150 mL) and concentrated hydrochloric acid (50 mL) was stirred for 12 hours. The liberated dibenzoyl-d-tartaric acid was filtered and dried (20 g). The filtrate was concentrated under reduced pressure to remove all the solvent. The residue obtained was stirred with acetone (15 ml x 2), filtered and dried to obtain colorless solid L-tert-leucine hydrochloride salt. It was suspended in toluene (50 mL) and epichlorohydrin (5.8 g) was added. The reaction mixture was stirred until the pH was neutral. It was filtered, the solid obtained was stirred with acetone (15 ml x 2) and again filtered to obtain L-tert-leucine (5.6 g, 75 % yield, 98.5 % chemical purity, 99.9% chiral purity). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping












