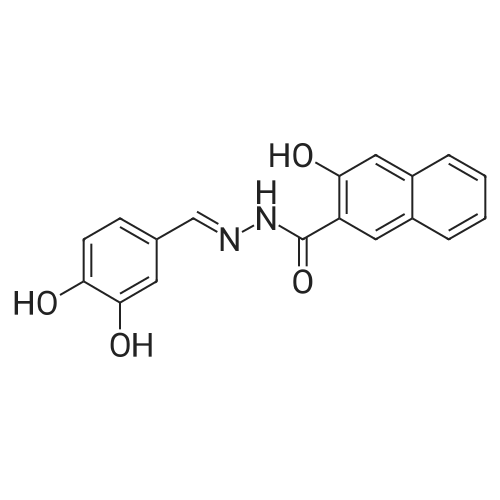
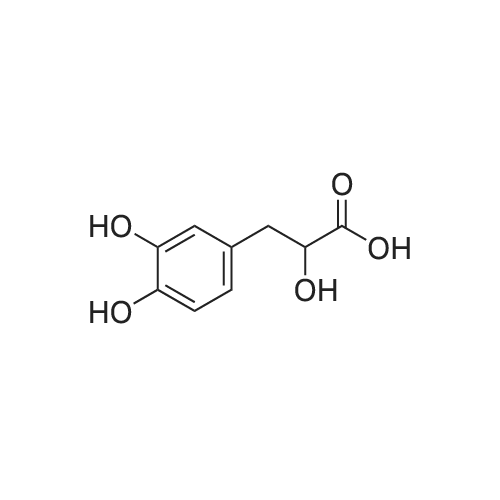
| 9.5 mg; 38.9 mg; 32.08 mg; 227.3 mg |
With sodium hydrogencarbonate; In water; at 180℃; for 1.0h;pH 4.0; |
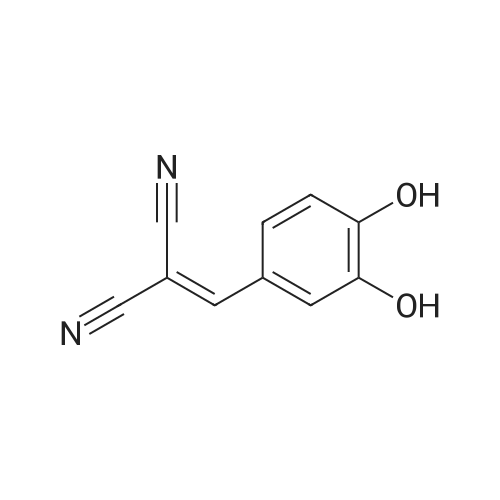
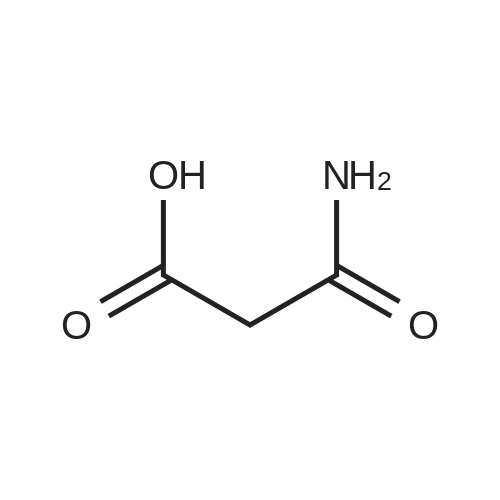
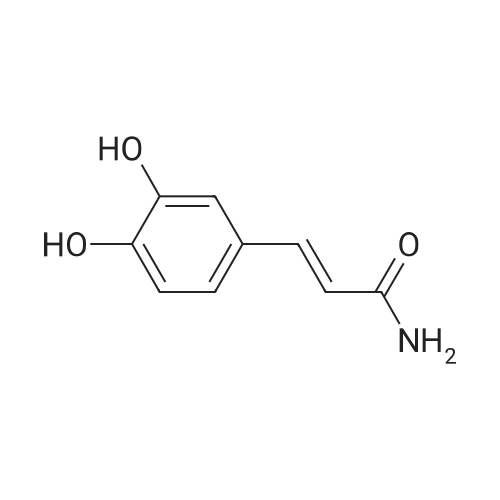
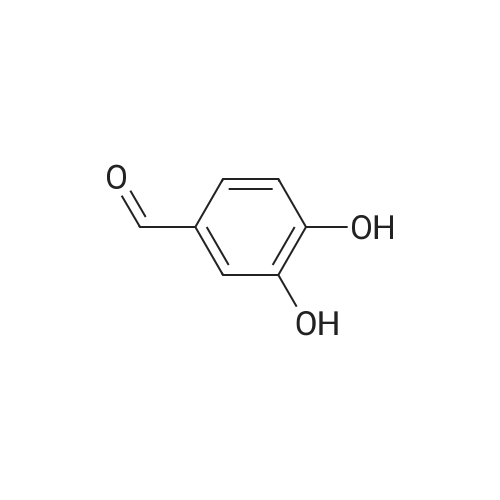
The salvianolic acid B (Fig. 3) was formulated with NaHCO3 water at pH 4.0 to prepare 40 mg / mL of salvianolic acid B solution,The solution was placed in a 50 mL subcritical water stainless steel reactor. After the furnace reached 180 C and stabilized, the reaction vessel was placed in a heating furnace and started to stand. 60min after the rapid removal of the reactor and into the ice bath cooling, the liquid removed, freeze-dried, rich in salvianolic acid A crude (Figure 4). 2. High-speed countercurrent chromatography separation purification salvianolic acid A The solvent system is petroleum ether: ethyl acetate: n-butanol: water=2:3: 1:9, add on 10 mm trifluoroacetic acid as stationary phase, down to 10 mm ammonia-water as the mobile phase, the high speed countercurrent chromatograph column volume is 300 ml, type quantity on 1.2g, speed 800 rpm, relative to stationary phase, down as the mobile phase, flow rate 2.0 ml/min, stationary phase retention rate of 57%, detection wavelength 280 nm. Specific operation steps are: according to the above-mentioned solvent proportion solvent system, arranged in the separatory funnel, layered after shaking, to balance after a period of time on the two-phase separated under, on adds together 10 mm trifluoroacetic acid as stationary phase, down to 10 mm ammonia-water as the mobile phase, taking 1.2g salvianolic acid A crude product rich in, dissolved in 5 ml plus 10 mm relative to the trifluoroacetic acid and 5 ml of in not ammonia down for use. Shanghai tauto Company is researching the semi-preparative high-speed countercurrent chromatograph, it is composed of a plunger pump, a sample introduction valve, ultraviolet detector, recorder and the chromatographic separation column (by the polytetrafluoroethylene tube multi-layer which is formed by winding of the spiral pipe, capacity of 300 ml) and the like, first of all make the sampling valve is in a sampling state, the fixed phase is pumped to a certain velocity of fully a chromatographic separation column, stop the pump. The opening of the speed controller, the high-speed flow chromatograph chromatographic separation column is rotary, speed up to 800 rpm when, the dissolved sample injector for injection counter current chromatograph injection valve the liquid storage tube, rotating sampling valve is the post state, the sample enter the chromatographic separation column. A mobile phase flow rate is 2.0 ml/min, mobile phase starts the pump, then according to the detector ultraviolet light (Figure 2) the spectrogram of the target component, shall be Radix Salviae Miltiorrhizae element (38.9 mg, Figure 5), salvianolic acid D (9.5 mg, Figure 6), salvianolic acid A (227.3 mg, Figure 7) and protocatechualdehyde (32.8 mg, Figure 8), HPLC analysis for the purity of 98% or more. The use of high performance liquid chromatography analysis isolate, liquid chromatography conditions: Kromasil 100 - 5C18 Column (4.6 × 250 mm), ultraviolet detection wavelength 286 nm, column temperature: 25 C, flow rate: 1.0 ml/min, the sample: 10muL, mobile phase using acetonitrile (A) and 0.2% formic acid aqueous solution (B) gradient elution, gradient conditions are as follows: 0 - 9min, 10% -22% A; 9 - 19min, 22% -24% A; 19 - 35min, 24% A; 35 - 43min, 24% -36% A; 43 - 48min, 36% -100% A; 48 - 50min, 100% A. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping