Alternatived Products of [ 139-65-1 ]

Product Citations
Systematic analysis of gut bacterial carcinogen metabolism and its functional consequences
Boyao Zhang
;
George-Eugen Maftei
;
Bartosz Bartmanski
, et al.
bioRxiv,2024:2024.05.20.595058.
DOI:
10.1101/2024.05.20.595058
More
Abstract: Organic carcinogens, in particular DNA-reactive compounds, contribute to the irreversible initiation step of tumorigenesis through introduction of genomic instability. Although carcinogen bioactivation and detoxification by human enzymes has been extensively studied, carcinogen biotransformation by human-associated bacteria, the microbiota, has not yet been systematically investigated. We tested the biotransformation of 68 mutagenic carcinogens by 34 bacterial species representative for the upper and lower human gastrointestinal tract and found that the majority (41) of the tested carcinogens undergo bacterial biotransformation. To assess the functional consequences of microbial carcinogen metabolism, we developed a pipeline to couple gut bacterial carcinogen biotransformation assays with Ames mutagenicity testing and liver biotransformation experiments. This revealed a bidirectional crosstalk between gut microbiota and host carcinogen metabolism, which we validated in gnotobiotic mouse models. Overall, the systematic assessment of gut microbiota carcinogen biotransformation and its interplay with host metabolism highlights the gut microbiome as an important modulator of exposome-induced tumorigenesis.
Purchased from AmBeed:
446-86-6 ;
121-66-4 ;
607-35-2 ;
67-20-9 ;
59-87-0 ;
117-39-5 ;
57-97-6 ;
5131-60-2 ;
512-56-1 ;
62-44-2 ;
6959-48-4 ;
84-65-1 ;
137-17-7 ;
117-39-5 ;
153-78-6 ;
1614-12-6 ;
298-81-7 ;
320-67-2 ;
99-55-8 ;
94-52-0 ;
101-61-1 ;
103-33-3 ;
114-83-0 ;
64091-91-4 ;
53-96-3 ;
3817-11-6 ;
90-94-8 ;
613-13-8 ;
56-57-5 ;
91-64-5 ;
26148-68-5 ;
101-80-4 ;
139-65-1 ;
366-70-1 ;
389-08-2 ;
99-59-2 ;
132-32-1 ;
105650-23-5 ;
394-69-4 ;
3544-23-8 ;
389-08-2 ;
320-67-2 ;
404-86-4 ;
82-28-0 ;
2832-40-8 ;
2475-45-8 ;
129-15-7
...More

Product Details of [ 139-65-1 ]
| CAS No. : | 139-65-1 |
MDL No. : | MFCD00025342 |
| Formula : |
C12H12N2S
|
Boiling Point : |
- |
| Linear Structure Formula : | NH2C6H4SC6H4NH2 |
InChI Key : | ICNFHJVPAJKPHW-UHFFFAOYSA-N |
| M.W : |
216.30
|
Pubchem ID : | 8765 |
| Synonyms : |
|
Application In Synthesis of [ 139-65-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 139-65-1 ]
- 1
-
 [ 628-36-4 ]
[ 628-36-4 ]

-
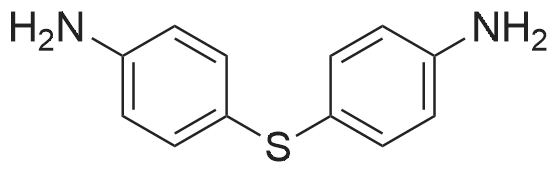 [ 139-65-1 ]
[ 139-65-1 ]

-
bis(4-(4H-1,2,4-triazol-4-yl)phenyl)sulfane
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
at 150℃; for 24h; |
The molar ratio of 4- (4-aminophenylthio) aniline: bisformylhydrazide was 1: 4In a 50 mL three-neck round bottom flask equipped with a magnetic stirrer, a reflux condenser and a thermometer, respectively4- (4-aminophenylthio) aniline (1 mmol)And bisformylhydrazide (4 mmol),Start stirring at 150oC,For 24 hours.After completion of the reaction,The reaction solution was cooled to room temperature,The resulting precipitate was added to 100 mL of hot methanol,After stirring and dissolving,filter,The filtrate was slowly evaporated to give a purple solid in 85percent yield. 4- (4-aminophenylthio) aniline bisformamide.In the invention, the molar ratio of 4- (4-aminophenylthio) aniline and bisformylhydrazine is 1: 4; the reaction temperature is 150 ,The reaction time was 24 hours. The organic compound was prepared by heating a solution of 4- (4-aminophenylthio) aniline and bisformylhydrazine using a "one-pot" process,Bis (4- (4H-1,2,4-triazol-4-yl) phenyl) sulfur (L). |
| 85% |
at 150℃; for 24h; |
The molar ratio of 4- (4-aminophenylthio) aniline: bisformylhydrazide was 1: 4 4- (4-aminophenylthio) aniline (1 mmol) and bisformylhydrazide (4 mmol) were added to a 50 mL three-necked round-bottomed flask equipped with a magnet, reflux condenser and thermometer,Start stirring at 150 oC and react for 24 hours.After the reaction, the reaction solution was cooled down to room temperature. The resulting precipitate was added to 100 mL of hot methanol, stirred and dissolved, and the filtrate was evaporated to give a colorless solid in a yield of 85percent.4- (4-aminophenylthio) aniline bisformamide. In the present invention, the molar ratio of 4- (4-aminophenylthio) aniline and bisformylhydrazine is 1: 4, the reaction temperature is 150 and the reaction time is 24 hours. The organic compound was prepared under heating using 4- (4-aminophenylthio) aniline and bisformylhydrazine using a "one pot" method. |
| 85% |
at 150℃; for 24h;Reflux; |
In the provided with a magneton, reflux condenser and a thermometer of the 50 ml round bottom flask in three separately adding 4 - (4 - amino-thio) aniline (1 mmol) and double-carbohydrazide (4 mmol), stir in 150oC, reaction 24 hours. After the reaction, the reaction liquid to room temperature, the obtained precipitation by adding 100 ml hot methanol, stirring to dissolve, filtering, the filtrate volatilize slow to get colorless solid, yield 85percent. |
| 85% |
at 150℃; for 24h; |
The molar ratio of 4- (4-aminophenylthio) aniline: dibenzohydrazide is 1: 4In a 50 mL three-necked round bottom flask equipped with a magnet, a reflux condenser and a thermometer4- (4-aminophenylthio) aniline (1 mmol) anddoubleFormylHydrazine(4 mmol), and the mixture was stirred at 150 ° C for 24 hours. After completion of the reaction, the reaction solution was lowered to room temperature, and the resulting precipitate was added to 100 mL of hot methanol. After stirring, the mixture was filtered and the filtrate was slowly evaporated to give a colorless solid in 85percent yield; 4- (4-aminophenylthio) aniline dicarboxylic acid hydrazide. |
|
at 150℃; for 48h; |
4-(4-aminophenylsulfanyl)aniline : 1,2-<strong>[628-36-4]diformylhydrazine</strong> molar ratio of 1:4 Equipped with a magnetic stirrer, reflux condenser and thermometer, a 50mL three-necked flask was added 4-(4-aminophenylsulfanyl)aniline (1mmol) and 1,2-<strong>[628-36-4]diformylhydrazine</strong> (4mmol). It was stirred at 150°C for 24 hours. After completion of the reaction, the reaction mixture was cooled to room temperature. The resulting precipitate was added to 100mL of hot methanol and stirred until dissolved. After, it was filtered and the filtrate slowly volatilize to give a purple solid, yield 85percent. Using the present invention of 4-(4-aminophenylsulfanyl)aniline : 1,2-<strong>[628-36-4]diformylhydrazine</strong> molar ratio of 1:4, it was reacted at 150°C for 24 hours in a "one-pot" reaction. 4-(4-aminophenylsulfanyl)aniline and 1,2-<strong>[628-36-4]diformylhydrazine</strong> under heating conditions prepared an organic compound bis(4-(4H-1,2,4-triazol-4-yl)phenyl)sulfide (L). |

Reference:
[1]Chemical Communications,2016,vol. 52,p. 3099 - 3102
[2]Patent: CN105418648,2016,A .Location in patent: Paragraph 0017; 0018
[3]Patent: CN106008567,2016,A .Location in patent: Paragraph 0013; 0014
[4]Patent: CN106187925,2016,A .Location in patent: Paragraph 0013; 0014; 0015
[5]Patent: CN106243156,2016,A .Location in patent: Paragraph 0013
[6]Patent: CN105348307,2016,A .Location in patent: Paragraph 0017; 0018; 0019
- 2
-
 [ 628-36-4 ]
[ 628-36-4 ]

-
 [ 139-65-1 ]
[ 139-65-1 ]

-
C12H12N2S*4C2H4N2O2
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
at 150℃; for 24h; |
Within three 50mL round bottom flask equipped with a magnetic sub, reflux condenser, and thermometer were added 4- (4-phenylsulfanyl) aniline (1 mmol of) andBis formic hydrazide (4mmol), stirring was started at 150oC, for 24 hours. After completion of the reaction, the reaction solution was cooled to room temperature, the resulting precipitate was added to 100mL of hot methanol, and stirred to dissolve, filtered, and the filtrate was slowly volatilize give a purple solid in 85percent yield. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping











