Alternatived Products of [ 139-33-3 ]
Product Details of [ 139-33-3 ]
| CAS No. : | 139-33-3 |
MDL No. : | MFCD00070672 |
| Formula : |
C10H14N2Na2O8
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
336.21
|
Pubchem ID : | - |
| Synonyms : |
Ethylenediaminetetraacetic acid disodium salt;EDTA-Na2;Edta disodium;Disodium edetate;Edetate disodium
|
Application In Synthesis of [ 139-33-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 139-33-3 ]
- 1
-
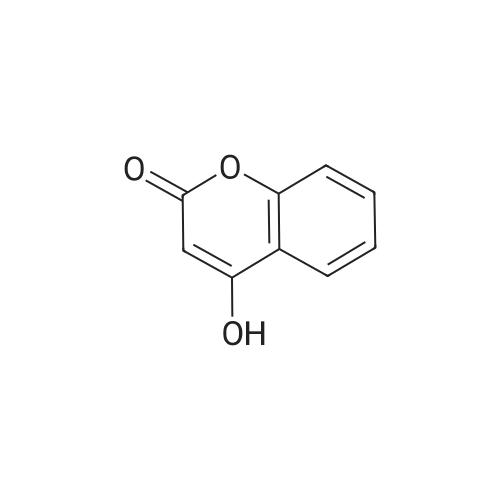 [ 1076-38-6 ]
[ 1076-38-6 ]

-
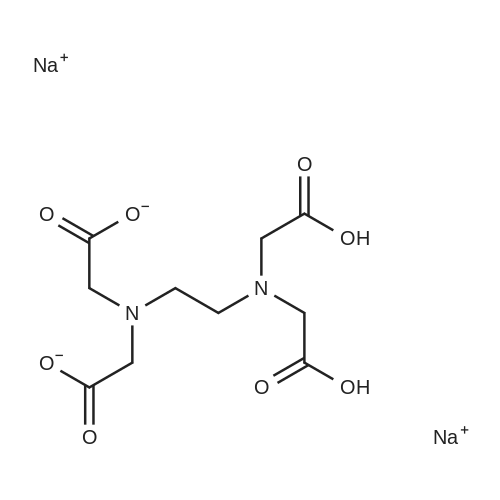 [ 139-33-3 ]
[ 139-33-3 ]

-
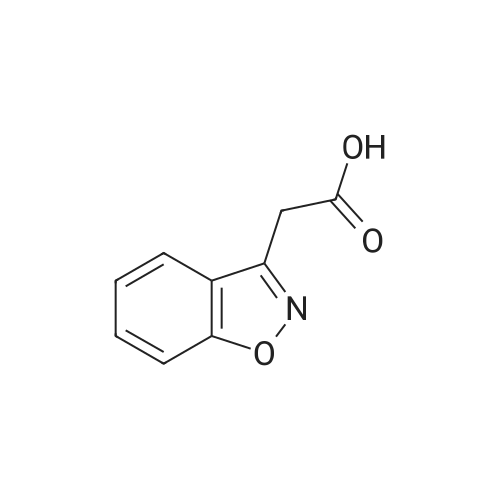 [ 4865-84-3 ]
[ 4865-84-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
EXAMPLE 1 1) A mixture of hydroxylamine sulfate (43 g), water (113 ml) and a 25% aqueous sodium hydroxide solution (57 ml) is stirred, and thereto are added 4-hydroxycoumarin (21 g) and ethylenediamine-tetraacetic acid disodium salt dihydrate (0.4 g), and the mixture is stirred with heating at 84 C. to 86 C. for 4 hours. The reaction mixture is cooled, and thereto are added 1,2-dichloroethane (30 ml) and water (43 ml), and the mixture is stirred. The 1,2-dichloroethane layer is removed, and the pH value thereof is adjusted to pH 1-2 with 62.5% sulfuric acid. The mixture is extracted twice with 1,2-dichloroethane (120 ml and 10 ml) to give a mixture of 1,2-benzisoxazole-3-acetic acid and 1,2-dichloroethane. The purity of 1,2-benzisoxazole-3-acetic acid in this mixture is 98%.; EXAMPLE 2 A mixture of hydroxylamine sulfate (344.0 g), water (904 ml) and a 25% aqueous sodium hydroxide solution (456 ml) is stirred, and thereto are added 4-hydroxycoumarin (168.0 g) and ethylenediaminetetraacetic acid disodium salt dihydrate (3.2 g), and the mixture is stirred with heating at 84 C.-86 C. for 4 hours. The reaction mixture is cooled, and thereto is added 1,2-dichloroethane (240 ml). The mixture is stirred, and the aqueous layer is collected. The pH value of the aqueous layer is adjusted to pH 1-2 with 25% sulfuric acid, and the precipitated crystals are collected by filtration, washed with water, and dried with air at 60 C. for 15 hours to give 1,2-benzisoxazole-3-acetic acid (170.4 g). |
| Yield | Reaction Conditions | Operation in experiment |
|
|
[0184] The shampoo compositions illustrated in the following Examples illustrate specific embodiments of the shampoo compositions of the present invention, but are not intended to be limiting thereof. Other modifications can be undertaken by the skilled artisan without departing from the spirit and scope of this invention. These exemplified embodiments of the shampoo composition of the present invention provide enhanced conditioning benefits to the hair. [0185] The shampoo compositions illustrated in the following Examples are prepared by conventional formulation and mixing methods, an example of which is set forth hereinbelow. All exemplified amounts are listed as weight percents and exclude minor materials such as diluents, preservatives, color solutions, imagery ingredients, botanicals, and so forth, unless otherwise specified. [0186] The compositions illustrated in the examples were prepared in the following manner (all percentages are based on weight unless otherwise specified). [0187] For each of the compositions, 6-9% of ammonium laureth-3 sulfate, P43 oil, PureSyn6 oil, cationic polymers, 0-1.5% Ammonium Xylene Sulfonate, and 0-5% water was added to a jacketed mix tank and heated to about 74 C. with agitation to form a solution. Citric Acid, Sodium Citrate, Sodium Benzoate, Disodium EDTA, Cocamide MEA and 0.6-0.9% Cetyl alcohol, were added to the tank and allowed to disperse. Ethylene glycol distearate (EGDS) was then added to the mixing vessel, and melted. After the EGDS was well dispersed (after about 10 minutes) preservative was added and mixed into the surfactant solution. This mixture was passed through a heat exchanger where it was cooled to about 35 C. and collected in a finishing tank. As a result of this cooling step, the ethylene glycol distearate crystallized to form a crystalline network in the product. [0188] Separately about 20% of the water was heated to about 74 C. and the remainder of the Cetyl Alcohol, Stearyl Alcohol, and the Cationic Surfactant were added to it. After incorporation, this mixture was passed through a heat exchanger where it was cooled to about 35 C. As a result of this cooling step, the Fatty Alcohols and surfactant crystallized to form a crystalline gel network. [0189] These two premixes are the mixed together and the remainder of the surfactants, perfume, Dimethicone, Sodium Chloride or Ammonium Xylene Sulfonate for viscosity adjustment and the remainder of the water were added with ample agitation to insure a homogeneous mixture. [0190] Preferred viscosities range from about 5000 to about 9000 centipoise at 27 C. (as measured by a Wells-Brookfield model RVTDCP viscometer using a CP-41 cone and plate at 2/s at 3 minutes). |
| Yield | Reaction Conditions | Operation in experiment |
|
|
EXAMPLES [0184] The shampoo compositions illustrated in the following Examples illustrate specific embodiments of the shampoo compositions of the present invention, but are not intended to be limiting thereof. Other modifications can be undertaken by the skilled artisan without departing from the spirit and scope of this invention. These exemplified embodiments of the shampoo composition of the present invention provide enhanced conditioning benefits to the hair. [0185] The shampoo compositions illustrated in the following Examples are prepared by conventional formulation and mixing methods, an example of which is set forth hereinbelow. All exemplified amounts are listed as weight percents and exclude minor materials such as diluents, preservatives, color solutions, imagery ingredients, botanicals, and so forth, unless otherwise specified. [0186] The compositions illustrated in the examples were prepared in the following manner (all percentages are based on weight unless otherwise specified). [0187] For each of the compositions, 6-9% of ammonium laureth-3 sulfate, P43 oil, PureSyn6 oil, cationic polymers, 0-1.5% Ammonium Xylene Sulfonate, and 0-5% water was added to a jacketed mix tank and heated to about 74 C. with agitation to form a solution. Citric Acid, Sodium Citrate, Sodium Benzoate, Disodium EDTA, Cocamide MEA and 0.6-0.9% Cetyl alcohol, were added to the tank and allowed to disperse. Ethylene glycol distearate (EGDS) was then added to the mixing vessel, and melted. After the EGDS was well dispersed (after about 10 minutes) preservative was added and mixed into the surfactant solution. This mixture was passed through a heat exchanger where it was cooled to about 35 C. and collected in a finishing tank. As a result of this cooling step, the ethylene glycol distearate crystallized to form a crystalline network in the product. [0188] Separately about 20% of the water was heated to about 74 C. and the remainder of the Cetyl Alcohol, Stearyl Alcohol, and the Cationic Surfactant were added to it. After incorporation, this mixture was passed through a heat exchanger where it was cooled to about 35 C. As a result of this cooling step, the Fatty Alcohols and surfactant crystallized to form a crystalline gel network. [0189] These two premixes are the mixed together and the remainder of the surfactants, perfume, Dimethicone, Sodium Chloride or Ammonium Xylene Sulfonate for viscosity adjustment and the remainder of the water were added with ample agitation to insure a homogeneous mixture. [0190] Preferred viscosities range from about 5000 to about 9000 centipoise at 27 C. (as measured by a Wells-Brookfield model RVTDCP viscometer using a CP-41 cone and plate at 2/s at 3 minutes). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping









