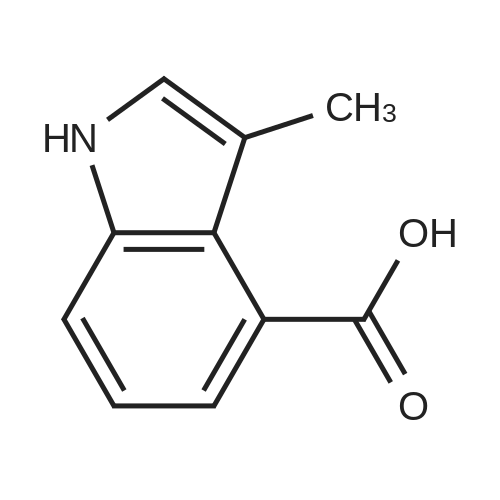
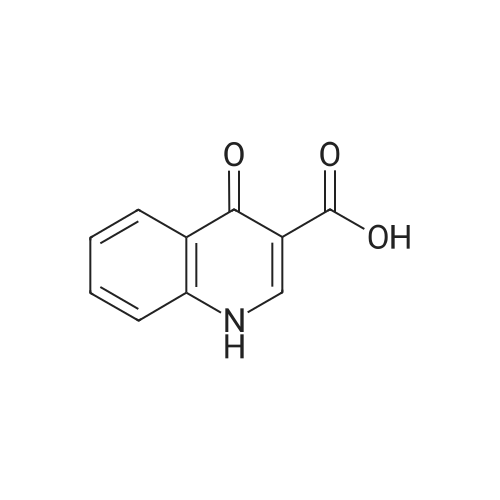
|
With hydrogenchloride; water; at 85 - 90℃; for 6.5h;Product distribution / selectivity; |
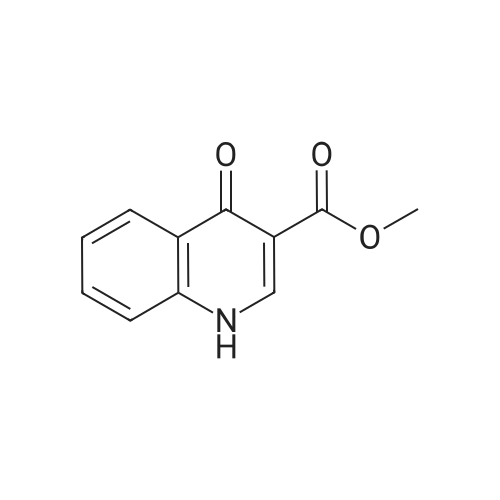
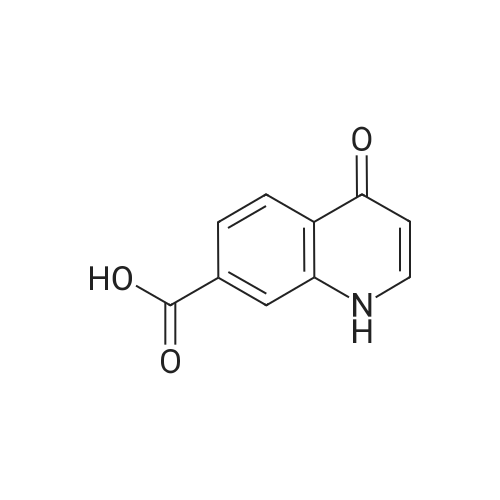
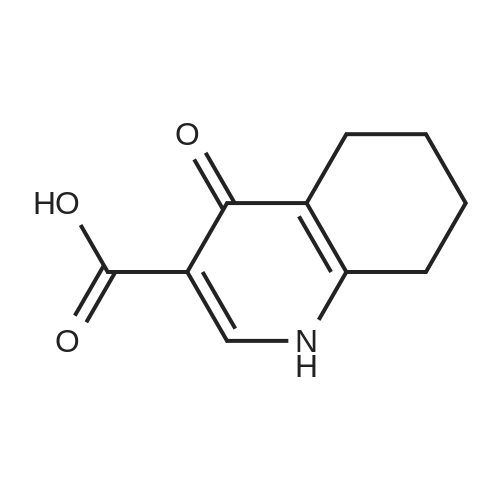
Compound 25 (1.0 eq) was suspended in a solution of HCl (10.0 eq) and H2O (11.6 vol). The slurry was heated to 85 - 90 0C, although alternative temperatures are also suitable for this hydrolysis step. For example, the hydrolysis can alternatively be performed at a temperature of from about 75 to about 100 C. In some instances, the hydrolysis is performed at a temperature of from about 80 to about 95 0C. In others, the hydrolysis step is performed at a temperature of from about 82 to about 93 C (e.g., from about 82.5 to about 92.5 C or from about 86 to about 89 0C). After stirring at 85 - 90 0C for approximately 6.5 hours, the reaction was sampled for reaction completion. Stirring may be performed under any of the temperatures suited for the hydrolysis. The solution was then cooled to 20 - 25 0C and filtered. The reactor/cake was rinsed with H2O (2 vol x 2). The cake was then washed with 2 vol H2O until the pH >; 3.0. The cake was then dried under vacuum at 60 0C to give compound 26. |
|
|
Compound 25 (11.3 g, 52 mmol) was added to a mixture of 10% NaOH (aq) (10 mL) and ethanol (100 mL). The solution was heated to reflux for 16 hours, cooled to 20-25 0C and then the pH was adjusted to 2-3 with 8% HCl. The mixture was then stirred for 0.5 hours and filtered. The cake was washed with water (50 mL) and then dried in vacuo to give compound 26 as a brown solid. 1H NMR (DMSO-d6; 400 MHz) delta 15.33 (s), delta 13.39 (s), delta 8.87 (s), delta 8.26 (m), delta 7.87 (m), delta 7.80 (m), delta 7.56 (m). |
|
|
Procedure for the preparation of 4-oxo-1,4-dihydroquinoline-3-carboxylic acid (26) Method 2Compound 25 (11.3 g, 52 mmol) was added to a mixture of 10% NaOH (aq) (10 mL) and ethanol (100 mL). The solution was heated to reflux for 16 hours, cooled to 20-25 C. and then the pH was adjusted to 2-3 with 8% HCl. The mixture was then stirred for 0.5 hours and filtered. The cake was washed with water (50 mL) and then dried in vacuo to give compound 26 as a brown solid. 1H NMR (DMSO-d6; 400 MHz) delta 15.33 (s), delta 13.39 (s), delta 8.87 (s), delta 8.26 (m), delta 7.87 (m), delta 7.80 (m), delta 7.56 (m). |
|
With water; sodium hydroxide; In ethanol; for 16h;Reflux; |
Compound 25 (11.3 g, 52 mmol) was added to a mixture of 10% NaOH (aq) (10 mL) and ethanol (100 mL). The solution was heated to reflux for 16 hours, cooled to 20-25 C and then the pH was adjusted to 2-3 with 8% HC1. The mixture was then stirred for 0.5 hours and filtered. The cake was washed with water (50 mL) and then dried in vacuo to give Compound 26 as a brown solid. 1H NMR (DMSO-d6; 400 MHz) delta 15.33 (s), delta 13.39 (s), delta 8.87 (s), delta 8.26 (m), delta 7.87 (m), delta 7.80 (m), delta 7.56 (m). |
|
With hydrogenchloride; water; at 85 - 90℃; |
Compound 25 (1.0 eq) was suspended in a solution of HQ (10,0 eq) and H20 (1 1.6 vol). The slum'' was heated to 85 - 90 C, although alternative temperatures are also suitable for this hydrolysis step. For example, the hydrolysis can alternatively be performed at a temperature of from about 75 to about 100 C. in some instances, fee hydrolysis is performed at a temperature of from about 80 to about 95 C. In others, the hydrolysis step is performed at a temperature of from about 82 to about 93 C (e.g., from about 82.5 to about 92.5 C or from about 86 to about 89 C). After stirring at 85 - 90 C for approximately 6.5 hours, fee reaction was sampled for reaction completion. Stirring may be performed under any of the temperatures suited for the hydrolysis. The solution was then cooled to 20 - 25 C and filtered. The reactor/cake was rinsed wife H2?> (2 vol x 2), The cake was then washed with 2 vol 0 until fee pH > 3.0, The cake was then dried under vacuum at 60 C to give Compound 26. |
|
With water; sodium hydroxide; In ethanol; for 16h;Reflux; |
Method 1[00326] Compound 25 (1.0 eq) was suspended in a solution ofHCl (10.0 eq) and H20 (11.6vol). The slurry was heated to 85 - 90 C, although alternative temperatures are also suitable forthis hydrolysis step. For example, the hydrolysis can alternatively be performed at a temperatureof from about 75 to about 100 C. In some instances, the hydrolysis is performed at atemperature of from about 80 to about 95 C. In others, the hydrolysis step is performed at atemperature of from about 82 to about 93 oc (e.g., from about 82.5 to about 92.5 oc or fromabout 86 to about 89 C). After stirring at 85 - 90 oc for approximately 6.5 hours, the reactionwas sampled for reaction completion. Stirring may be performed under any of the temperaturessuited for the hydrolysis. The solution was then cooled to 20 - 25 oc and filtered. Thereactor/cake was rinsed with H20 (2 vol x 2). The cake was then washed with 2 vol H20 untilthe pH 2: 3.0. The cake was then dried under vacuum at 60 octo give compound 26.Method 2[00327] Compound 25 (11.3 g, 52 mmol) was added to a mixture of 10% NaOH (aq) (10 mL)and ethanol (100 mL). The solution was heated to reflux for 16 hours, cooled to 20-25 oc andthen the pH was adjusted to 2-3 with 8% HCl. The mixture was then stirred for 0.5 hours andfiltered. The cake was washed with water (50 mL) and then dried in vacuo to give compound 26as a brown solid. 1H NMR (DMSO-d6; 400 MHz) 8 15.33 (s), 8 13.39 (s), 8 8.87 (s), 8 8.26 (m),8 7.87 (m), 8 7.80 (m), 8 7.56 (m). |
| 7.5 g |
With water; sodium hydroxide; at 80 - 85℃; for 3h; |
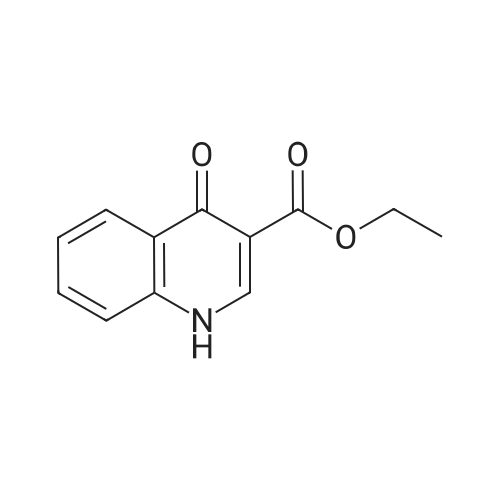
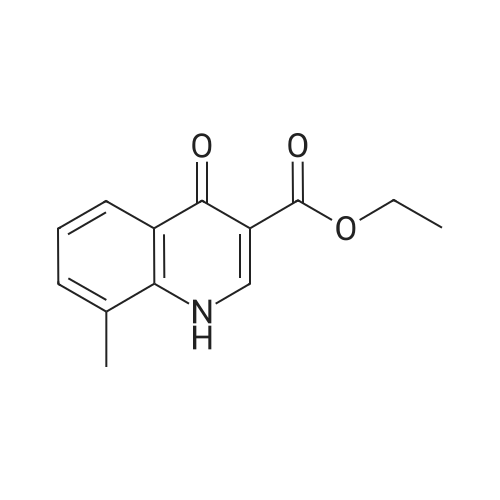
In a clean round bottom flask, ethyl 4-oxo-l, 4-dihydroquinoline-3-carboxylate (10.0 gm) was charged to a solution of sodium hydroxide (3.7 gm) in 13.0 ml water. The reaction mass was heated for 3.0 hr at 80-85 C and then cooled to 25-30. To this was added 0.10 gm of activated charcoal and filtered. The pH was adjusted using con HCL and the product was filtered and washed with water. The wet cake slurried in methanol at 25 -30 C and filtered. The product was dried under vacuum at 50.0 C to get 7.50 gm of title productPurity by HPLC - 99.75 % |
|
|
Compound 25 (11.3 g, 52 mmol) was added to a mixture of 10% NaOH (aq) (10 mL) and ethanol (100 mL). The solution was heated to reflux for 16 hours, cooled to 20-25 C. and then the pH was adjusted to 2-3 with 8% HCl. The mixture was then stirred for 0.5 hours and filtered. The cake was washed with water (50 mL) and then dried in vacuo to give Compound 26 as a brown solid. 1H NMR (DMSO-d6; 400 MHz) delta 15.33 (s), delta 13.39 (s), delta 8.87 (s), delta 8.26 (m), delta 7.87 (m), delta 7.80 (m), delta 7.56 (m). |
|
With hydrogenchloride; In water; at 85 - 90℃; |
Method 1 [00341] Compound 25 (1.0 eq) was suspended in a solution of HC1 (10.0 eq) and H20 (1 1.6 vol). The slurry was heated to 85 - 90 C, although alternative temperatures are also suitable for this hydrolysis step. For example, the hydrolysis can alternatively be performed at a temperature of from about 75 to about 100 C. In some instances, the hydrolysis is performed at a temperature of from about 80 to about 95 C. In others, the hydrolysis step is performed at a temperature of from about 82 to about 93 C (e.g., from about 82.5 to about 92.5 C or from about 86 to about 89 C). After stirring at 85 - 90 C for approximately 6.5 hours, the reaction was sampled for reaction completion. Stirring may be performed under any of the temperatures suited for the hydrolysis. The solution was then cooled to 20 - 25 C and filtered. The reactor/cake was rinsed with H20 (2 vol x 2). The cake was then washed with 2 vol H20 until the pH > 3.0. The cake was then dried under vacuum at 60 C to give compound 26. |
|
With sodium hydroxide; at 90 - 100℃; |
Step c : 4-Oxo 1,4-dihydroquinoline- 3-carboxylic acid 4-hydroxyquinoline 3- carboxylic acid ethyl ester (100 g) was suspended in 2N sodium hydroxide solution at room temperature and was heated to 90-100 C. and maintained for 2-4 hours. After completion, the reaction mass was cooled to room temperature and filtered to remove undissolved material. The obtained filtrate was acidified to pH 3-4 with 2N Hydrochloric acid at 25-30 C. The resultant solid was filtered, washed with water and dried at 50 C. until constant weight was observed to obtain the title compound (55-65 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping