| 16% |
|
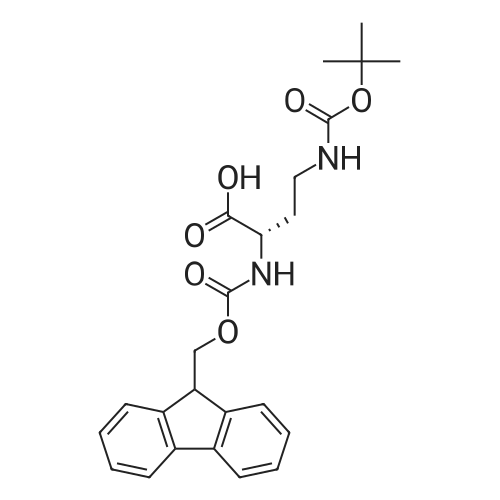
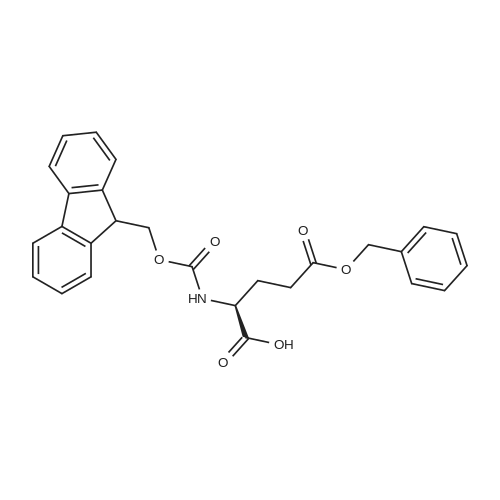
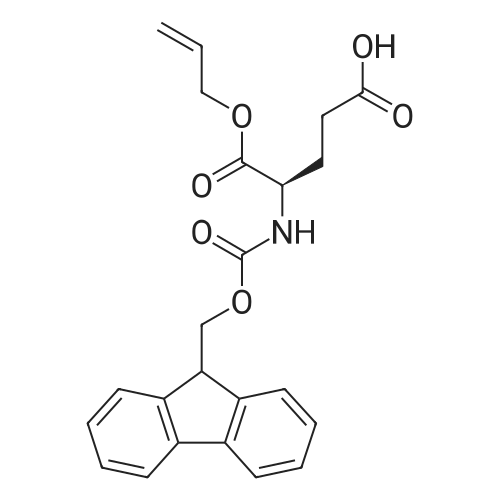
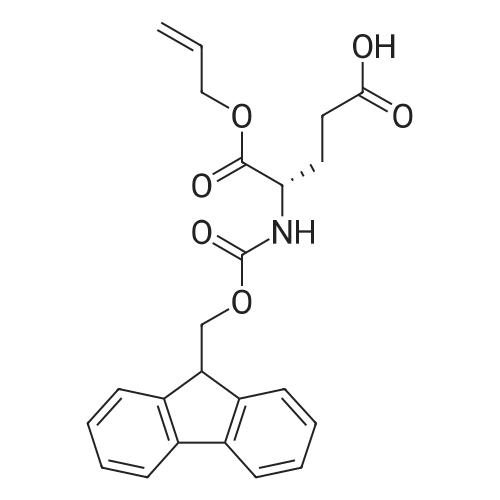
6.1 Specific Peptides. (0263) Peptides of the following structures were synthesized by the general methods described above, and except where indicated MC1-R Ki and MC4-R Ki values for each peptide were determined in competitive binding assays using [I125]-NDP-alpha-MSH as described in 7.1 below. All peptides were prepared in the TFA acid salt form, except for the peptides of Examples 43, 46 and 67, which were prepared in the acetate salt form. (0264) The syntheses of some specific peptides of the invention are illustrated below. These peptides were prepared using solid phase peptide synthesis by means of a Symphony Multiplex Peptide Synthesizer (Rainin Instrument Company/Protein Technologies Inc) automated peptide synthesizer. Step 1: Coupling of Orn (0265) The Sierber resin 9-Fmoc-Aminoxanthen-3-yloxy-polystyrene resin (0.39 mol/g, ChemPep Inc., 151902) was swelled in 3×5 mL of DMF for 10 min. Thereafter, Fmoc was deprotected using 2×5 mL of 20% piperidine in DMF for 10 min. The resin was then washed in 6×5 mL DMF for 30 sec. 5 mL of 200 mM <strong>[147290-11-7]<strong>[147290-11-7]Fmoc-Orn(Alloc)</strong>-OH</strong> in DMF and 5 mL 200 mM HBTU containing 400 mM NMM in DMF was added and after 30 min the resin was washed with 3×5 mL DMF for 30 sec. Step 2: Coupling of Next 6 Amino Acids (AA) (0266) The resin from step 1 was first swelled in 3×5 mL of DMF for 30 sec, Fmoc was deprotected using 2×5 mL of 20% piperidine in DMF for 10 min and then washed with 6×5 mL DMF for 30 sec. 5 mL of 200 mM Fmoc-AA-OH solution and 5 mL 200 mM HBTU containing 400 mM NMM in DMF was added and after 30 min the resin was washed with 3×5 mL DMF for 30 sec. (0267) This step was repeated for each amino acid (AA). Step 3: Acetylation (0268) Fmoc was deprotected using 2×5 mL of 20% piperidine in DMF for 10 min and the resin was then washed with 3×5 mL DMF for 30 sec. Thereafter, 5 mL of 50% Ac2O/DMF solution was added and after 30 min the peptide resin was washed with 3×5 mL DMF for 30 sec and 6×5 mL DCM for 30 sec. Step 4: Allyl/Alloc Deprotection (0269) The peptide resin (0.6 mmol) was mixed with phenylsilane (Oakwood Chemical, S13600) (20 eq.) in 20 mL of DCM and bubbled with nitrogen for 5 min. (0270) Tetrakis(triphenylphosphine)-palladium(0) (Strem Chemicals, Inc., 46-2150) (0.2 eq.) was added and the mixture was agitated with nitrogen for 1 hour. The procedure was repeated one time for 1 hour and an additional time for 30 min with fresh reagents. The treated peptide resin was then washed with DCM x 3 and DMF x 3. Step 5: Lactam Formation (0271) The lactam ring was formed on the peptide resin using TBTU (2 eq.) and ethyldiisopropylamine (DIEA) (4 eq.) in 20 mL DMF for 1 hour. A second coupling may be needed if a positive Kaiser Ninhydrin test is observed. Step 6: Peptide Cleavage (0272) The peptide resin (0.6 mmol) was mixed with 20 mL of 5% sodium diethyldithio-carbamate trihydrate (NaCS2NEt2, Aldrich, 228680) in DMF for 20 min and then washed with DMF×3, DCM×3 and diethyl ether×2. (0273) The resin (0.6 mmol) was then stirred in a 25 mL of TFA/TIS/H2O (90:5.0:5.0 v/v/v) for 2.5 hours. The resin was filtered. The filtrate was concentrated to about 10 mL in volume and about 140 mL of cold diethyl ether (pre-cooled to about 0 C.) was added. (0274) The mixture was vortexed, and then placed in the refrigerator (about -4 C.) for 1 h, centrifuged for 5 min at 2800 rpm, and the ether layer was decanted. (0275) The peptide was washed with 90 mL of cold diethyl ether (pre-cooled to about 0 C.), vortexed, centrifuged for 5 min at 2800 rpm, and ether layer decanted. (0276) The resulting solid was dissolved in 50% AcOH/H2O and stored at room temperature overnight. (0277) The crude peptide solution was concentrated to afford solid crude peptide for HPLC purification. (0278) After HPLC purification, the peptide TFA salt was converted to peptide acetate salt using ion exchange (×100 eq.). The anion exchange resin used was Dowex SBR LC NG, OH-form (Supelco, Cat14036-U). Example 67 (0284) [table-us-00009-en] (SEQIDNO:72) Ac-Arg-cyclo(Glu-Pro-D-Phe(4-F)-Arg-Trp-Orn)-NH2 (0285) The procedure described above was followed in the preparation of the title peptide except for that 15 mL of 5% sodium diethyldithio-carbamate trihydrate in DMF, 16 mL of TFA/TIS/H2O and 90 mL+60 mL of diethyl ether were used in step 6. Moreover, the filtrate was concentrated to 5 mL. (0286) The amino acids added in step 2 were, in the order of being coupled, Fmoc-Trp(Boc)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-D-Phe(4-F)-OH, Fmoc-Pro-OH, Fmoc-Glu(OAll)-OH, and Fmoc-Arg(Pbf)-OH. (0287) The resulting peptide was purified by HPLC (column: Atlantis dC18 OBD 19×100 mm (5mu., Waters part 186001367) using 10% MeOH/H2O containing 0.1% TFA (solvent A) and 90% MeOH/H2O containing 0.1% TFA (solvent B). A gradient of 5%-10% of solvent B for 5 min and 10%-40% of solvent B for 30 min was used. (0288) The peptide yield was 16%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping