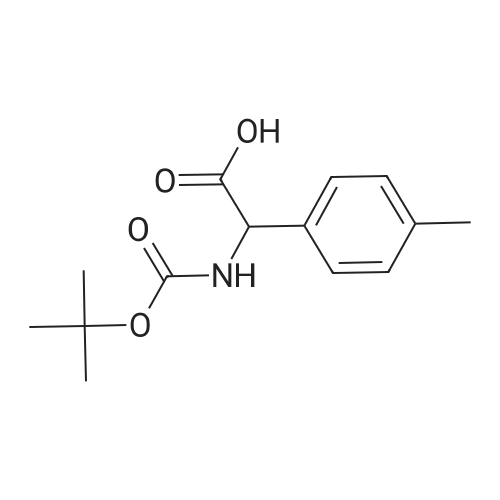
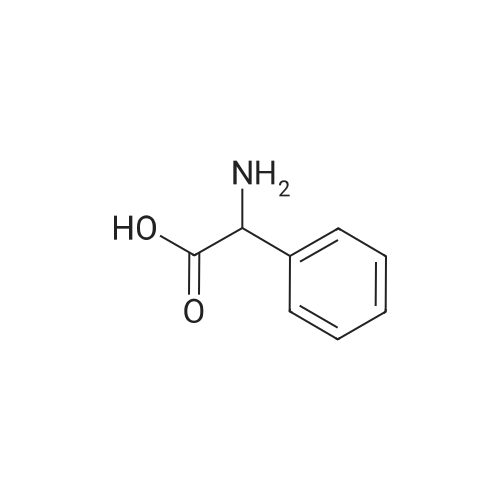
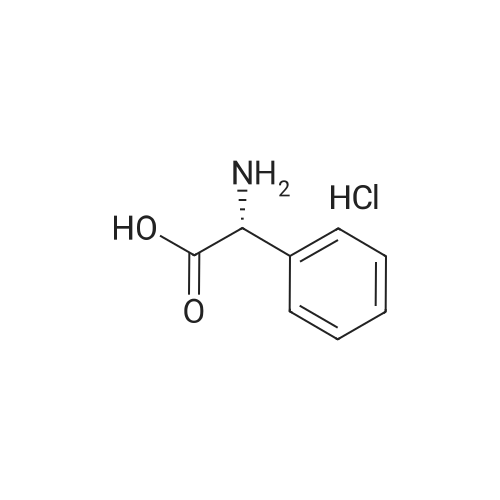
| 95% |
With sodium hydroxide; In 1,4-dioxane; at 20℃; |
A one-necked round-bottom flask was charged with amino(4-methylphenyl)acetic acid (10 g, 60.5 mmol), 1,4- dioxane (300 mE) and 1M NaOH (170 mE). To the resulting solution di-tert-butyl dicarbonate (19.8 g, 90.8 mmol) was added and the solution stirred at room temperature overnight. The dioxane was removed under reduced pressure and the aqueous solution was adjusted to pH 4 with 3M HC1 and the product extracted with ethyl acetate (2x50 mE). The combined organic layers were dried over Na2SO4, filtered and the solvent removed under reduced pressure to afford [(tert-butoxycarbonyl)amino](4-methylphenyl)acetic acid as a white solid (15.2 g, y=95%). ‘H NMR (DMSO-d5) ? (ppm): 7.44 (d, 1H, J=7.4 Hz), 7.27 (d, 2H, J=7.2 Hz), 7.14 (d, 2H, J=7.3 Hz),5.03 (d, 1H, J=5.4 Hz), 2.28 (s, 3H), 1.38 (s, 9H). MS (ES’) mlz: 266.42 (M+1). A one-necked round-bottom flask was charged with [(tert-butoxycarbonyl)amino](4-methylphenyl) acetic acid (16 g, 60.5 mmol), DCM (500 mE) and triethylamine (16.7 mE, 121 mmol). The resulting solution wascooled to 0 C. and N-(3-dimethylaminopropyl)-N-ethylcar-bodiimide hydrochloride (EDC, 13.9 g, 72.6 mmol) and 1 -hydroxybenzotriazole (HOBT, 9.8 g, 72.6 mmol) were added.The mixture was stirred for 40 mm at 0 C. Then 1 -(tetrahydrothran-2-yl)methanamine (10.6 mE, 103 mmol) was addedand the solution stirred at room temperature overnight. After18 h the reaction was complete (HPEC-MS analysis). Thesolution was washed with water (2x 150 mE), 1M HC1 (2x 150mE), and brine (2x1 50 mE). The organic layers were driedover Na2SO4, filtered and the solvent was removed underreduced pressure to afford a white solid which was taken up in100 mE of n-hexane and triturated at room temperature for 2h. The precipitate was filtered and dried under vacuum at 50C. for 4 h. The solid was dissolved in 100 mE of 1 ,4-dioxaneand 25 mE of 4M HC1 solution in dioxane were added. Theresulting solution was stirred at room temperature overnight.Afier solvent removal under vacuum, the residue was takenup in diethyl ether (40 mE) and triturated at room temperaturefor 2 h. The precipitate was filtered and dried under vacuum at50 C. to afford Intermediate C as a white solid (11.6 g,y=67% over two steps). ‘H NMR (DMSO-d5) ? (ppm): 8.75(br s, 3H), 7.45 (d, 2H, J=7.5 Hz), 7.22 (d, 2H, J=7.4 Hz), 4.91(br s, 1H), 3.90-3.51 (m, 4H), 3.22-3.04 (m, 2H), 2.28 (s, 3H),1.85-1.26 (m, 4H). MS (ES’) mlz: 249.04 (M+1). |
| 80% |
With sodium hydroxide; In tetrahydrofuran; water; at 20℃; for 15h; |
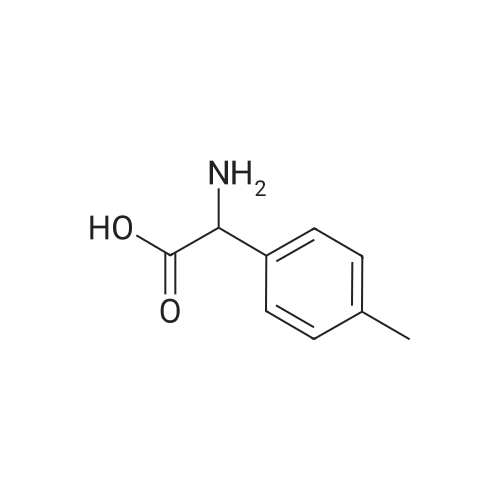
To a suspension of 2-amino-2-p-tolylacetic acid (1.00 g, 6.05 mmol) in THF (30 ml) and water (30 ml), was added 2N sodium hydroxide (30.3 ml, 60.5 mmol) and di-tert-butyl dicarbonate (2.64 g, 12.1 mmol). The reaction was stirred at RT for 15 hours, and then THF was evaporated. The remaining aqueous phase was cooled and acidified with 37% HCl until pH 1. The desired compound was extracted with EtOAc, and the organic phase was washed with brine, dried over Na2SO4 and evaporated to obtain 2-(tert-butoxycarbonylamino)-2-p-tolylacetic acid (1.29 g; 80% yield). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping