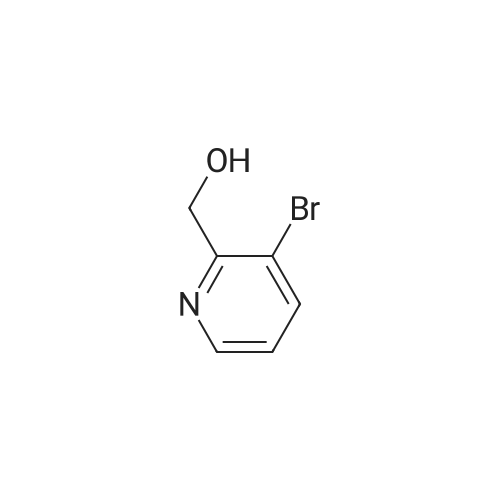
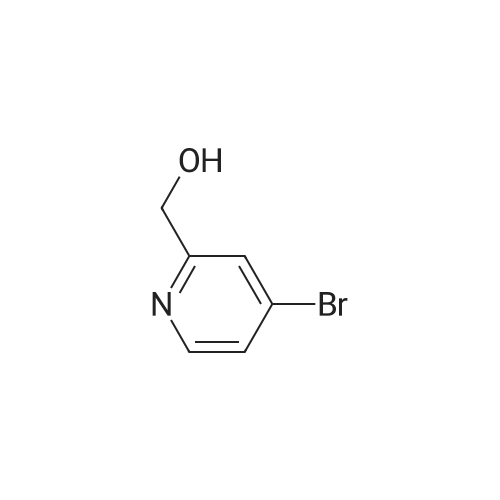
| 100% |
With manganese(IV) oxide; In chloroform; for 0.75h;Reflux; |
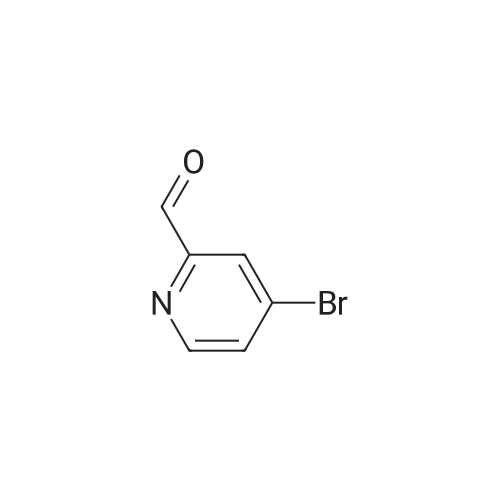
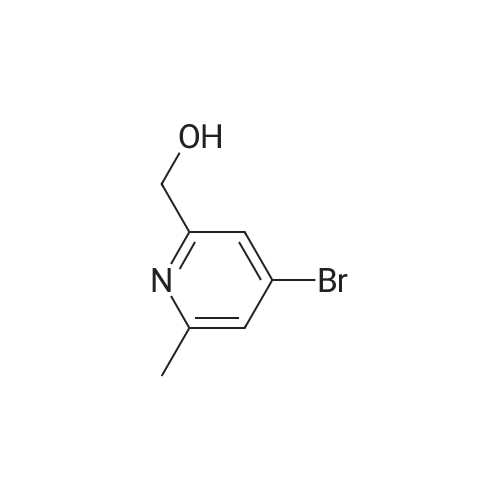
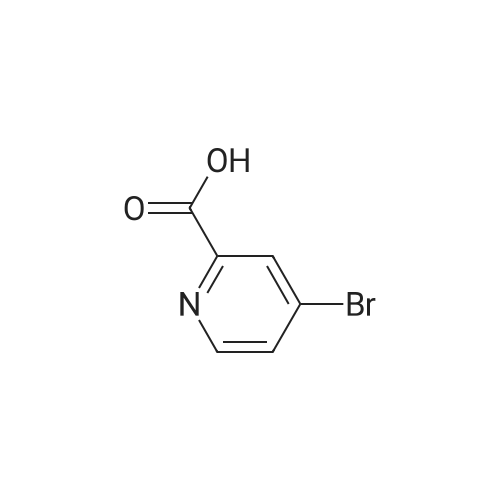
Example 29i 4-Bromopicolinaldehyde Manganese(IV) oxide (22.19 g, 255.29 mmol) was added to a solution of (4-bromopyridin-2-yl)methanol (4.00 g, 21.27 mmol) in chloroform (80 mL) and the reaction mixture was stirred under reflux for 45 min. After the mixture had cooled to room temperature the solids were removed by filtration through a pad of Celite.(R).. The solvent was removed in vacuo and the residue (3.96 g, quant.) was used without further purification in the next step. 1H NMR (500 MHz, DMSO-d6) delta ppm 9.97 (s, 1H) 8.80 (d, 1H) 7.98 (d, 1H) 7.88 (dd, 1 H); MS (APCI+): m/z 186, 188 [M+H]+. |
| 75% |
With oxalyl dichloride; dimethyl sulfoxide; triethylamine; In dichloromethane; at -65 - -60℃; for 1.5h;Inert atmosphere; |
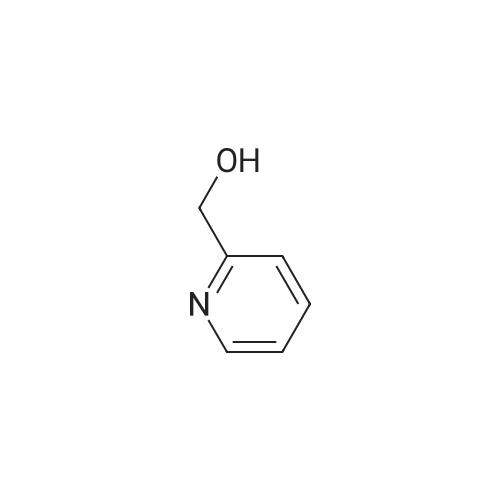
Under the protection of nitrogen,Add 3L DCM to the 5L three-neck bottle to cool down.240g of oxalyl chloride was added dropwise during cooling;At -60 ° C,295.6 g of Dimethyl sulfoxide (DMSO) was added dropwise to the reaction solution.Keep warm for 30min;At -60 ° C,237.5 g of Cpd 3 was added dropwise to the reaction solution.The reaction was carried out at -65 ° C for 1 hour;At this temperature, 3.5 eq of triethylamine (TEA) was added dropwise.After the solution was allowed to stand, the plate was measured.Through the column, the product 4-bromopyridine-2-carbaldehyde is obtained.(Cpd 4) 177.2g,The yield was 75percent. |
|
|
Description 5: 4-Bromo-2-pyridinecarbaldehyde (D5); To a solution of the oxalyl chloride (0.364 mL, 4.09 mmol) in dry DCM (30 mL) at -780C was added DMSO (0.634 mL, 8.93 mmol) in dry DCM (5 mL) dropwise. The mixture was stirred at this temperature under argon atmosphere for 10 minutes <n="29"/>before a solution of (4-bromo-2-pyridinyl)methanol (D4) (700 mg, 3.72 mmol) in dry DCM (15 ml.) was added dropwise. After circa 30 minutes, triethylamine (2.6 mL, 18.6 mmol) was added and the cooling bath was removed. The reaction mixture was allowed to stir at room temperature for 1 h after which water was added and it was extracted 3 times with DCM. The combined organics were dried over MgSO4. The crude material (700 mg) was purified by flash chromatography (Biotage SP4, 25+M column) with a gradient of EtOAc in hexane to afford 425 mg (61 percent) of the desired product D5.1H-NMR (CDCI3): delta 7.70 (1 H, dd), 8.12 (1 H1 d), 8.61 (1 H, d), 10.05 (1 H, s); Description 5 - alternative procedure: 4-Bromo-2-pyridinecarbaldehyde (D5); To a solution of oxalyl chloride (13.2 mL, 0.149 mol) in dry dichloromethane (1 L) at - 78 0C under argon was added a solution of DMSO (23 mL, 0.324 mol) in dry dichloromethane (180 mL) dropwise over 20 minutes. The mixture was stirred at -78 0C under argon for 15 minutes and then a solution of (4-bromo-2-pyridinyl)methanol (D4) (25.4 g, 0.135 mol) in dry dichloromethane (500 mL) was added dropwise over 30 minutes. The resulting white suspension was stirred at -78 CC for 40-45 minutes and then triethylamine (95 mL, 0.676 mol) was added dropwise over 15 minutes. After stirring at -78 0C for 15 minutes, the mixture was allowed to reach room temperature over -1.5 h and then poured into water (400 mL). The organic layer was separated and the aqueous layer was extracted with dichloromethane (3x, ~1 L total solvent). The combined organic layers were washed with brine (20OmL), dried over MgSO4 and concentrated. The crude material was purified by flash chromatography on silica gel with a gradient of 0 to 30 percent ethyl acetate in hexane to afford 20.5 g (82percent) of the desired product D5, with NMR data consistent with those previously obtained. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping