|
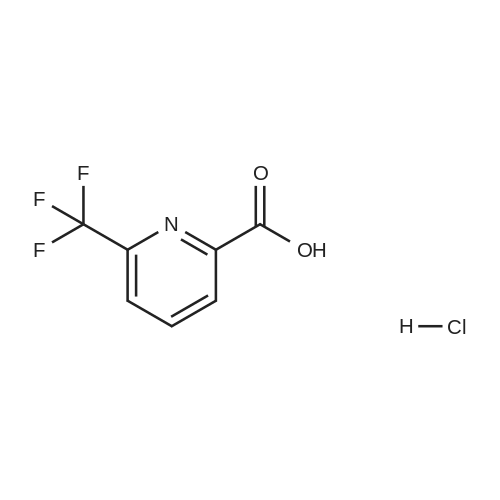
With hydrogenchloride; In water; at 80℃; for 48h; |
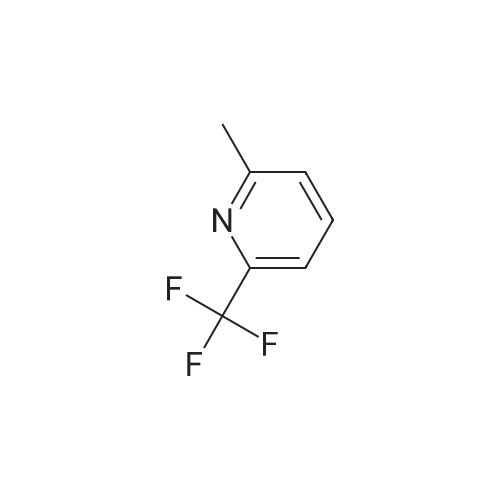
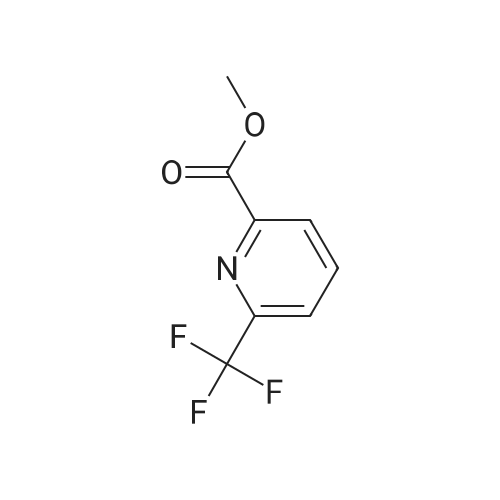
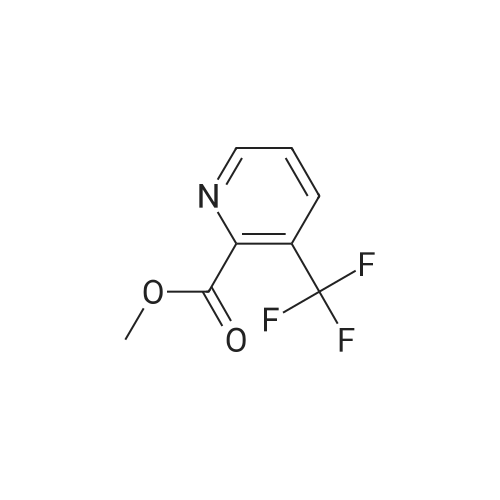
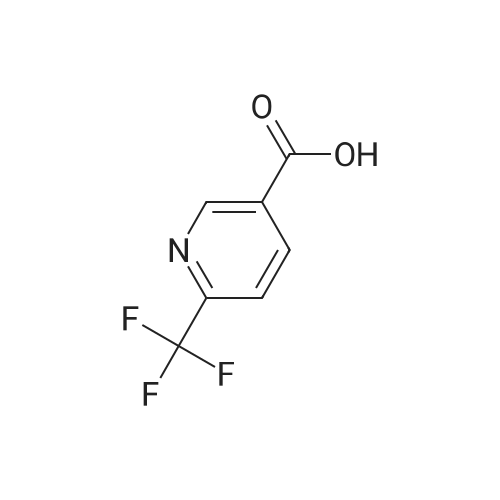
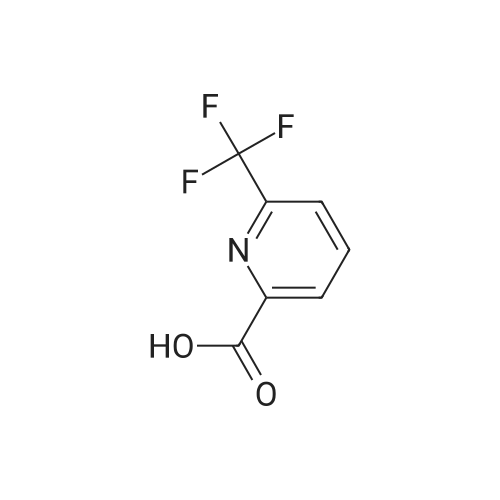
To a solution of 6-trifluoromethyl-pyridine-2-carboxylic acid in methanol (770 ml) was added concentrated HC1 (6 ml). The mixture was stirred at 80C for 48 hours then concentrated to remove the volatile.The crude product was diluted with ethyl acetated and washed with Sat. NaHCO3 solution. Theorganic layer was dried with anhydrous Na2504 and concentrated to give6-trifluoromethyl-pyridine-2-carboxylic acid methyl ester (10) as a white solid. LC-MS: m/z 206(M+H). |
|
With acetyl chloride; at 45 - 70℃;Inert atmosphere; |
Methanol was added to the reaction vessel under nitrogen atmosphere. 6-trifluoromethyl-pyridine-2-carboxylic acid (150 g, 0.785 mol) was added and dissolved at ambient temperature. Acetyl chloride (67.78 g, 0.863 mol) was added dropwise at a temperature below 45C. The reaction mixture was maintained at 65-70C for about 2-2.5 h, and then concentrated at 3 5-45C under vacuum and cooled to 25-35C. The mixture was diluted with ethyl acetate and rinsed with saturated NaHCO3 solution then rinsed with brine solution. The mixture was concentrated at 3 5-45C under vacuum and cooled to 25-35C, then rinsed with nheptane and concentrated at 3 5-45C under vacuum, then degassed to obtain brown solid, which was rinsed with n-heptane and stirred for 10-15 minute at 25-35C. The suspension was cooled to -40 to -3 0C while stirring, and filtered and dried to provide 6-trifluoromethyl-pyridine-2- carboxylic acid methyl ester. |
| 21 g |
With sulfuric acid; for 14h;Reflux; |
To a solution of 6-trifluoromethyl-pyridine-2-carboxylic acid (22 g, 1 equiv.) in methanol (200 mL) was added concentrated H2S04 (0.3 mL). The mixture was stirred at reflux for 14 hours and then cooled to ambient. Solid NaHCO3 (10 g) was added and the suspension was stirred for 30 minutes. The mixture was filtered and the filtrate was concentrated to remove the volatile. The crude product was diluted with ethyl acetated (200 mL) and dried with anhydrous Na2504. The mixture was filtered and concentrated to give 6-trifluoromethyl-pyridine-2-carboxylic acid methyl ester (2) as a waxy solid (21 g). |
|
With acetyl chloride; at 45 - 70℃;Inert atmosphere; |
Methanol was added to the reaction vessel under nitrogen atmosphere. 6-trifluoromethyl-pyridine-2-carboxylic acid (150 g, 0.785 mol) was added and dissolved at ambient temperature. Acetyl chloride (67.78 g, 0.863 mol) was added dropwise at a temperature below 45 C. The reaction mixture was maintained at 65-70 C. for about 2-2.5 h, and then concentrated at 35-45 C. under vacuum and cooled to 25-35 C. The mixture was diluted with ethyl acetate and rinsed with saturated NaHC03 solution then rinsed with brine solution. The mixture was concentrated at 35-45 C. under vacuum and cooled to 25-35 C., then rinsed with n-heptane and concentrated at 35-45 C. under vacuum, then degassed to obtain brown solid, which was rinsed with n-heptane and stirred for 10-15 minute at 25-35 C. The suspension was cooled to -40 to -30 C. while stirring, and filtered and dried to provide 6-trifluoromethyl-pyridine-2-carboxylic acid methyl ester. |
|
With sulfuric acid; at 65℃; for 10h; |
[1344] to a solution of 6-(trifluoromethyl)picolinic acid (10 g, 52.33 mmol) in MeOH (150 ml) was added H2SO4 (1.03 g, 10.47 mmol, 557.88 ul) dropwise. After stirred at 65 C for 10 hours, the mixture was cooled to room temperature, neutralized with a saturated aqueous NaHCO3 solution, and extracted with ch2c12 (70 ml x 3). The organic phases were combined, dried with anhydrous Na2SO4, and evaporated to afford crude intermediate compound 287a (9.20 g, 85.71% yield) as white solid. 1H NMR (400 mhz, CDCl3) delta 8.32 (d, = 8.0 hz, 1h), 8.09 - 8.05 (m, 1h), 7.88 (d, = 8.0 hz, 1h), 4.03 (s, 3h). |
|
With acetyl chloride; at 45 - 70℃;Inert atmosphere; |
Example 2, Step 2: Preparation of 6-trifluoromethyl-pyridine-2-carboxylic acid methyl ester Methanol was added to the reaction vessel under nitrogen atmosphere. 6-trifluoromethyl-pyridine-2-carboxylic acid (150 g, 0.785 mol) was added and dissolved at ambient temperature. Acetyl chloride (67.78 g, 0.863 mol) was added dropwise at a temperature below 45 C. The reaction mixture was maintained at 65-70 C. for about 2-2.5 h, and then concentrated at 35-45 C. under vacuum and cooled to 25-35 C. The mixture was diluted with ethyl acetate and rinsed with saturated NaHCO3 solution then rinsed with brine solution. The mixture was concentrated at 35-45 C. under vacuum and cooled to 25-35 C., then rinsed with n-heptane and concentrated at 35-45 C. under vacuum, then degassed to obtain brown solid, which was rinsed with n-heptane and stirred for 10-15 minute at 25-35 C. The suspension was cooled to -40 to -30 C. while stirring, and filtered and dried to provide 6-trifluoromethyl-pyridine-2-carboxylic acid methyl ester. |
|
With thionyl chloride; for 12h;Reflux; |
6-Trifluoromethylpyridine-2-carboxylic acid (25 g, 130.8 mmol) was dissolved in 300 mL of methanol.Thionyl chloride(23.3 g, 196.2 mmol), the mixture was heated and refluxed for 12 h. The reaction solution is concentrated and dried.Add saturated sodium bicarbonate solution to adjust the pH, and extract with ethyl acetate.Dry over anhydrous sodium sulfate and concentrate to give the title compound. |
|
With thionyl chloride; for 12h;Reflux; |
6-Trifluoromethylpyridine-2-carboxylic acid (25 g, 130.8 mmol) was dissolved in 300 mL of methanol, and thionyl chloride (23.3 g, 196.2 mmol) was added dropwise. After addition, the mixture was refluxed for reaction for 12 h. The resultant solution was concentrated untill dry, and saturated sodium hydrogen carbonate solution was added to adjust the pH, and then the resultant was extracted with ethyl acetate, dried over anhydrous sodium sulfate to give the title compound. |
|
With thionyl chloride; for 12h;Reflux; |
6-trifluoromethylpyridine-2-carboxylic acid(25g, 130.8mmol) dissolved in 300mL of methanol,Thionyl chloride (23.3 g, 196.2 mmol) was added dropwise.The mixture was heated to reflux for 12 h. The reaction solution is concentrated and dried.Add saturated sodium bicarbonate solution to adjust the pH,Extracted with ethyl acetate and dried over anhydrous sodium sulfate.Concentrated to give the title compound. |
|
With thionyl chloride; for 12h;Reflux; |
6-Trifluoromethylpyridine-2-carboxylic acid (25 g, 130.8 mmol) was dissolved in 300 mL of methanol, and thionyl chloride (23.3 g, 196.2 mmol) was added dropwise, and the mixture was refluxed for 12 h. The reaction solution is concentrated and dried.Add the saturated sodium bicarbonate solution to adjust the pH, extract with ethyl acetate and dry over anhydrous sodium sulfate.Concentrated to give the title compound. |
|
With thionyl chloride; at 12℃;Reflux; |
6-Trifluoromethylpyridine-2-carboxylic acid (25 g, 130.8 mmol) was dissolved in 300 mL of methanol.Thionyl chloride (23.3 g, 196.2 mmol) was added dropwise, and the mixture was heated under reflux for 12 h.The reaction mixture was concentrated to dryness.ConcentratedTitle compound. |
|
With thionyl chloride; for 12h;Reflux; |
6-Trifluoromethylpyridine-2-carboxylic acid (25 g, 130.8 mmol) was dissolved in 300 mL of methanol, and thionyl chloride (23.3 g, 196.2 mmol) was added dropwise, and the mixture was refluxed for 12 h. The reaction mixture was concentrated to dryness. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping