Alternatived Products of [ 13086-84-5 ]
Product Details of [ 13086-84-5 ]
| CAS No. : | 13086-84-5 |
MDL No. : | MFCD00995019 |
| Formula : |
C8H19O3P
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
194.21
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 13086-84-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 13086-84-5 ]
- 1
-
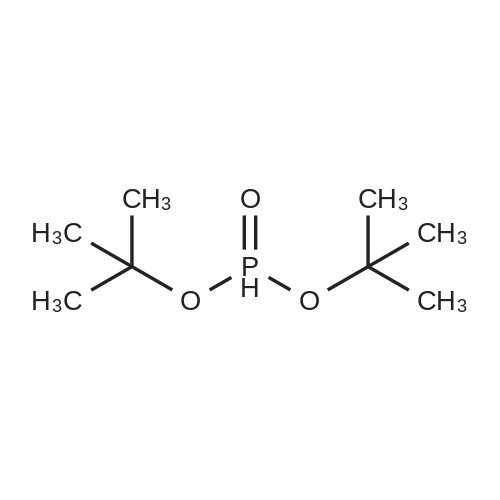 [ 13086-84-5 ]
[ 13086-84-5 ]

-
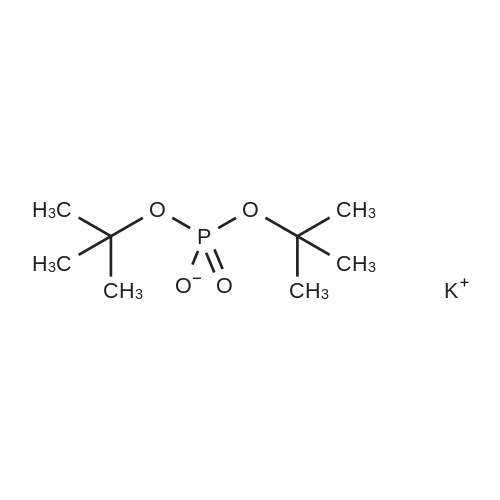 [ 33494-80-3 ]
[ 33494-80-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With potassium permanganate; potassium hydrogencarbonate; In water; at 5 - 20℃; for 19h; |
Intermediate 59: Bis(1,1 -dimethylethyl) hydrogen phosphate potassium salt; A solution of bis(1 ,1-dimethylethyl) hydrogen phosphite (100 g, 515 mmol, Alfa Aesar) and potassium bicarbonate (30.9 g, 309 mmol) in water (800 ml) was cooled to 5 0C in an ice bath. To this mixture, potassium permanganate (57.0 g, 360 mmol) was added portionwise over 1 h maintaining the temperature below 20 0C. The reaction mixture was stirred at ambient temperature for 18 h. The reaction was heated to 60 0C and then filtered slowly through a pad of celite. The filtrate was evaporated in vacuo to give the title compound as a white solid (109.7 g); 1H NMR (d6-DMSO) delta 1.26 (18 H, s). |
- 2
-
 [ 13086-84-5 ]
[ 13086-84-5 ]

-
 [ 33494-80-3 ]
[ 33494-80-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With potassium permanganate; potassium hydrogencarbonate; In water; at 0 - 20℃; for 1.5h; |
The conversion of di-tert-butyl phosphite into the corresponding phosphate is performed by a slight modification of the method published by Zwierzak and Kluba (Zwierzak, A and Kluba, M., 1971). Di-tert-butyl phosphite (40.36 mmole) is combined with potassium bicarbonate (24.22 mmole) in 35 ml of water. The solution is stirred in an ice bath and potassium permanganate (28.25 mmole) is added in three equal portions over one hour's time. The reaction is then allowed to continue at room temperature for an additional half hour. Decolorizing carbon (600 mg) is then incorporated as the reaction is heated to 60°C for 15 minutes. The reaction is then vacuum filtered to remove solid magnesium dioxide. The solid is washed several times with water. The filtrate is then combined with one gram of decolorizing carbon and heated at 60°C for an additional twenty minutes. The solution is again filtered to yield a colorless solution, to which a slight excess of concentrated HCl is slowly added wich efficient stirring in an ice bath. The addition of acid causes the precipitation of the di-tert-butyl phosphate free acid. The free acid is then filtered and washed with ice cold water. The compound is then converted to the salt form by dissolving the free acid in acetone and adding an equal molar amount of tetramethylammonium hydroxide while keeping the reaction cooled by a salt/ice bath with efficient stirring. The resulting clear solution is placed under reduced pressure to give 7.16 grams of crude product. This product is then recrystallized by refluxing in dimethoxyethane and slow cooling at room temperature to give 6.52 g of pure product (57percent yield). 12.75 mmole of the tetramethylammonium di-tert-butyl-phosphate is then mixed with 70 ml of dimethoxyethane and brought to reflux. Twenty-five grams of chloroiodomethane is then added and stirred for one and a half hours. The reaction is then filtered and the filtrate is placed under reduced pressure to remove excess chloroiodomethane and solvent. The two products are then separated via flash column chromatography. The stationary phase is normal phase silica (30 g). The mobile phase consists of ethyl acetate and hexane in a 3 to 7 (v/v) ratio respectively. The chloromethyl di-tert-butyl phosphate is isolated as a pale gold oil (63percent yield): 1H NMR (CDCl3, 300 MHz) delta 1.51 (s, 12H), 5.63 (d, 2H, J = 14.8). Mass spectrum (FAB +, GLY) 259 (M+1). |
| 5 g |
|
Di-tert-butyl phosphohite (40.36 mmole) was combined with potassium bicarbonate (24.22 mmole) in 35 ml of water. The solution was stirred in an ice bath and potassium permanganate (28.25 mmole) was added in three equal portions over one hour's time. The reaction as then allowed to continue at room temperature for an additional half hour. Decolorizing carbon (600 mg) was then incorporated as the reaction was heated to 60° C. for 15 minutes. The reaction was then vacuum filtered to remove solid magnesium dioxide. The solid was washed several times with water. The filtrate was then combined with one gram of decolorizing carbon and heated at 60° C. for an additional twenty minutes. The solution was again filtered to yield a colorless solution, which was then evaporated under vacuum to afford crude Di-tert-butyl phosphate potassium salt. Di-tert-butyl phosphate potassium salt (5 g, 20.14 mmole) was dissolved in methanol (15 g): to this solution at 0° C. a slight excess of concentrated HCl is slowly added with efficient stirring at 0° C. The addition of acid causes the precipitation of potassium chloride. The solid is then filtered and washed with methanol. The compound in the mother liquor is then converted to the ammonium form by adding an equal molar amount of tetramethylammonium hydroxide (3.65 g, 20.14 mmole) while keeping the reaction cooled by a salt/ice bath with efficient stirring. The resulting clear solution is placed under reduced pressure to give the crude product. To the tetramethylammonium di-tert-butyl-phosphate dissolved in refluxing dimethoxyethane is then added 4.3 grams of chloroiodomethane (24.16 mmole) and stirred for 1-2 hours. The reaction is then filtered and the filtrate is placed under reduced pressure to concentrate the solution in DME. The chloromethyl di-tert-butyl phosphate 12-16percent in DME is used in the synthesis of 4-(5-(2-(3,5-bis(trifluoromethyl)phenyl)-N,2-dimethylpropanamido)-4-(o-tolyl)pyridin-2-yl)-1-methyl-1-((phosphonooxy)methyl)piperazin-1-ium without further purifications (60percent yield): 1HNMR (CD3OD, 300 MHz) delta 1.51 (s, 12H), 5.63 (d, 2H, J=14.8). 31P-NMR (CD3OD, 300 MHz) delta ?11.3 (s, 1P) |
|
With potassium permanganate; potassium hydrogencarbonate; In water; at 20℃; for 1.5h; |
Di-tert-butyl phosphohite (40.36 mmole) was combined with potassium bicarbonate (24.22 mmole) in 35 ml of water. The solution was stirred in an ice bath and potassium permanganate (28.25 mmole) was added in three equal portions over one hour's time. The reaction as then allowed to continue at room temperature for an additional half hour. (0203) Decolorizing carbon (600 mg) was then incorporated as the reaction was heated to 60° C. for 15 minutes. The reaction was then vacuum filtered to remove solid magnesium dioxide. The solid was washed several times with water. The filtrate was then combined with one gram of decolorizing carbon and heated at 60° C. for an additional twenty minutes. The solution was again filtered to yield a colorless solution, which was then evaporated under vacuum to afford crude Di-tert-butyl phosphate potassium salt. Di-tert-butyl phosphate potassium salt (5 g, 20.14 mmole) was dissolved in methanol (15 g): to this solution at 0° C. a slight excess of concentrated HCl is slowly added with efficient stirring at 0° C. The addition of acid causes the precipitation of potassium chloride. The solid is then filtered and washed with methanol. The compound in the mother liquor is then converted to the ammonium form by adding an equal molar amount of tetramethylammonium hydroxide (3.65 g, 20.14 mmole) while keeping the reaction cooled by a salt/ice bath with efficient stirring. The resulting clear solution is placed under reduced pressure to give the crude product. To the tetramethylammonium di-tert-butyl-phosphate dissolved in refluxing dimethoxyethane is then added 4.3 grams of chloroiodomethane (24.16 mmole) and stirred for 1-2 hours. The reaction is then filtered and the filtrate is placed under reduced pressure to concentrate the solution in DME. The chloromethyl di-tert-butyl phosphate 12-16percent in DME is used in the synthesis of 4-(5-(2-(3,5-bis(trifluoromethyl)phenyl)-N,2-dimethylpropanamido)-4-(o-tolyl)pyridin-2-yl)-1-methyl-1-((phosphonooxy)methyl)piperazin-1-ium without further purifications (60percent yield): 1HNMR (CD3OD, 300 MHz) delta 1.51 (s, 12H), 5.63 (d, 2H, J=14.8). 31P-NMR (CD3OD, 300 MHz) delta ?11.3 (s, 1P). |
|
With potassium permanganate; potassium hydrogencarbonate; In water; at 60℃; for 1.5h; |
Di-tert-butyl phosphite (40.36 mmole)Was combined with potassium bicarbonate (24.22 mmole) in 35 ml of water.The solution was stirred in an ice bath and potassium permanganate (28.25 mmole)Was added in three equal portions over 1 hour.Thereafter, the reaction was continued at room temperature for an additional 0.5 hour.Thereafter, decolorizing carbon (600 mg)The reaction was taken up when heated to 60 ° C. for 15 minutes.Thereafter, the reaction product was subjected to vacuum filtration to remove manganese dioxide as a solid component.The solid was washed several times with water. The filtrate was then combined with 1 gram of decolorizing carbon and heated at 60 ° C. for a further 20 minutes. The solution was again filtered to give a colorless solution which was then evaporated under vacuum to provide the crude di-tert-butyl phosphate potassium salt.Dissolve di-tert-butyl phosphate potassium salt (5 g, 20.14 mmole) in methanol (15 g) and add a slight excess of concentrated HCl to this solution at 0 ° C. while slowing the efficiency Stirring was carried out. The addition of acid causes the precipitation of potassium chloride. The solids are then filtered and washed with methanol. Thereafter, the compound in the mother liquor was treated with ammonium (3.65 g, 20.14 mmole) by adding an equimolar amount of tetramethylammonium hydroxide (3.65 g, 20.14 mmole) while efficiently stirring the reaction and cooling with a salt / ice bath Morphology. The resulting clear solution is placed under reduced pressure to provide a crude product.To tetramethylammonium di-tert-butyl-phosphate dissolved in refluxing dimethoxyethane, 4.3 grams of chloroiodomethane (24.16 mmole) is then added and stirred for 1-2 hours. The reaction is then filtered and the filtrate is placed under reduced pressure and the solution is concentrated in DME. 12 to 16percent of chloromethyl di-tert-butyl phosphate in DME4- (5- (2- (3,5-bis (trifluoromethyl) phenyl)-N, 2-dimethylpropanamido-4- (o-tolyl) pyridin-2-yl)-1-methyl-1 - ((phosphonooxy) methyl) piperazin-1-ium used without further purification (60percent yield): |


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







