|
|
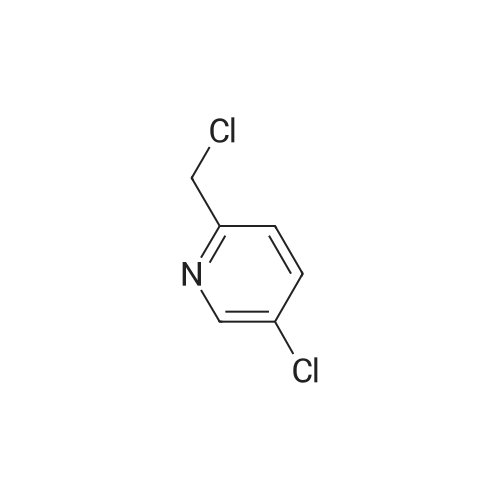
Preparation of 2-(((3,5-bis(trifluoromethyl)phenyl)thio)methyl)-5-chloropyridine (6A). Sodium hydroxide (2N, aqueous, 5.46 mL, 10.9 mmol) was added dropwise to a solution of 3,5-bis-trifluoromethyl benzenethiol (1.83 mL, 10.9 mmol) in MeOH (3 mL). This mixture was stirred for 5 minutes and then <strong>[10177-24-9]5-chloro-2-(chloromethyl)pyridine</strong> (1.77 g, 10.9 mmol) was added as a solution in MeOH (10 mL). This mixture was stirred 2 hours at r.t. and then it was concentrated to about half volume in vacuo. EtOAc and half saturated aqueous ammonium chloride were added, the layers were separated, and the aqueous layer was extracted with EtOAc (lx). The combined organic layers were dried over anhydrous MgSO/i, filtered, and concentrated in vacuo to give 2-(((3,5- bis(trifluoromethyl)phenyl)thio)methyl)-5-chloropyridine (6A) as a solid (4.1 g, 101% yield). MS m/z = 372 [M+H]+. NMR (400 MHz, CDC13) δ ppm 8.49 (d, J=2.35 Hz, 1 H) 7.75 (s, 2 H) 7.61 - 7.65 (m, 2 H) 7.32 (d, J=8.08 Hz, 1 H) 4.32 (s, 2 H). |
| 4.1 g |
|
Sodium hydroxide (5.4 mL of 2 N solution, 10.8 mmol) was added dropwise to a solution of 3,5-bis-trifluoromethyl benzenethiol ( Sigma- Aldrich Chemical Company, Inc., St. Louis, MO, USA) (1.8 mL, 11 mmol) in MeOH (3 mL) at room temperature. This mixture was stirred for 5 minutes and then <strong>[10177-24-9]5-chloro-2-(chloromethyl)pyridine</strong> (Sigma-Aldrich Chemical Company, Inc., St. Louis, MO, USA) (1.8 g, 11 mmol) was added as a solution in MeOH (10 mL). This mixture was stirred for 2 hours at room temperature and then it was concentrated to about half volume in vacuo. EtOAc and half saturated aqueous ammonium chloride were added, the layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over anhydrous MgSO/t, filtered, and concentrated in vacuo to give 2-(((3,5-bis(trifluoromethyl)phenyl)thio)methyl)-5- chloropyridine (8a) (4.1 g, 101% yield) as a solid. MS m/z = 372 [M+H]+. NMR (400 MHz, CDC13) δ ppm 8.49 (d, J = 2.35 Hz, 1H) 7.75 (s, 2H) 7.61 - 7.65 (m, 2H) 7.32 (d, J = 8.08 Hz, 1H) 4.32 (s, 2H). |
|
With sodium hydroxide; In methanol; water; at 20℃; for 2h; |
Sodium hydroxide (2 N, aqueous, 5.4 mL, 10.8 mmol) was added dropwise to a solution of 3,5-bis-trifluoromethyl benzenethiol (Sigma-Aldrich Chemical Company, Inc., St. Louis, MO, USA) (1.8 mL, 10.9 mmol) in MeOH (3 mL). This mixture was stirred for 5 min and then <strong>[10177-24-9]5-chloro-2-(chloromethyl)pyridine</strong> (Sigma-Aldrich Chemical Company, Inc., St. Louis, MO, USA) (1.77 g, 10.9 mmol) was added as a solution in MeOH (10 mL). This mixture was stirred for 2 h at RT and then it was concentrated to about half volume in vacuo. EtOAc and half saturated aqueous ammonium chloride were added, the layers were separated, and the aqueous layer was extracted with EtOAc (1 x). The combined organic layers were dried over anhydrous MgSO4, filtered, and concentrated in vacuo to give 2-(((3,5-bis(trifluoromethyl)phenyl)thio)methyl)-5-chloropyridine (33a) as a solid (4.1 g, 101% yield). MS m/z = 372 [M+H]+. 1H NMR (400 MHz, CDCl3) ^ ^ppm 8.49 (d, J = 2.35 Hz, 1 H) 7.75 (s, 2 H) 7.61 - 7.65 (m, 2 H) 7.32 (d, J = 8.08 Hz, 1 H) 4.32 (s, 2 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +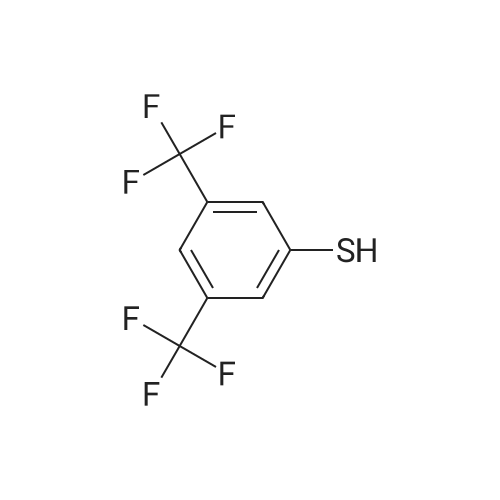

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping