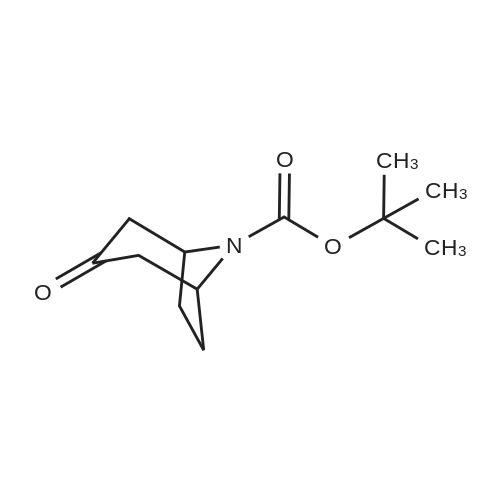
| 88% |
With N-ethyl-N,N-diisopropylamine; In dichloromethane; at 20℃; for 0.5h; |
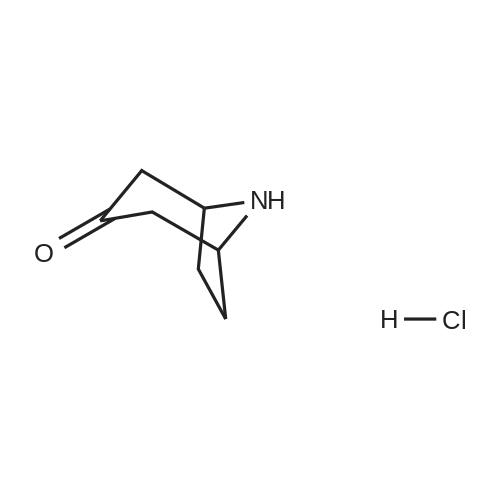
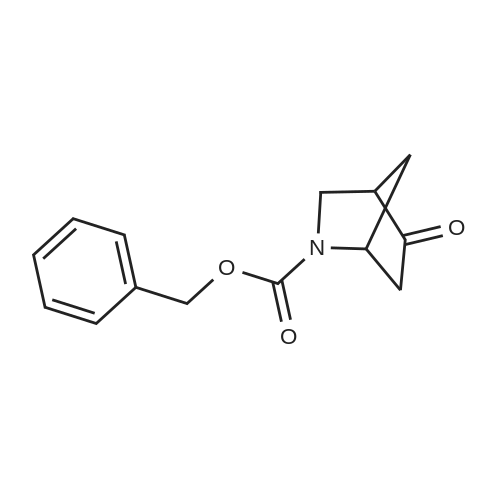
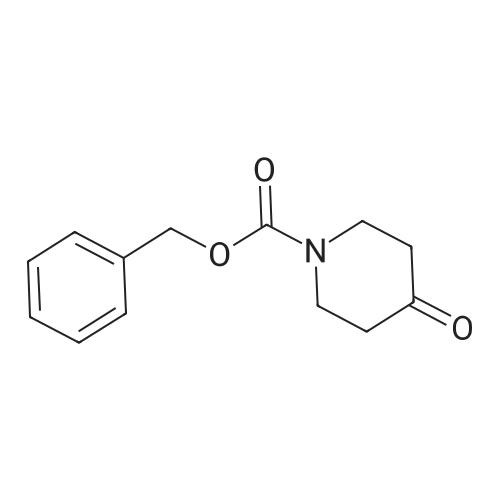
Tropinone (10.0 g; 71.84 mmol) was dissolved in DCE (60 mL) and treated drop-wisewith 1-chloroethyl chloroformate ACE-C1 (14.5 mL; 19.11 g; 133.7 mmol). The reaction wasallowed to stir at room temperature overnight and was then diluted with Et20 (400 mL) andfiltered. The filtrate was concentrated under reduced pressure to provide the crude chloroethylcarbamate. This compound was taken in MeOH (200 mL) and stirred at room temperature for 1h, then concentrated under reduced pressure (at 55°C) to provide the crude des-methyltropinoneas the HC1 salt (tan solid, 11.4 g, 98percent yield). The crude material was recrystallized fromacetonitrile to furnish the pure product as a white crystalline solid (5 g, 43percent yield). *H NMR(400 MHz, DMSO-d6) 8 1.79 (dd, J= 15.0, 6.9 Hz, 2H), 2.09 (m, 2H), 2.40 (d, J= 16.7 Hz,2H), 3.02 (dd, J= 17.1, 4.3 Hz, 2H), 4.23 (s, 2H), 10.00 (br s, 2H)Des-methyl tropinone (5.10 g; 31.55 mmol) was dissolved in CH2CI2 (50 mL) and treated withbenzyl chloroformate (4.29 mL; 5.11 g; 29.98 mmol) DIPEA (16.48 mL; 12.23 g; 94.66 mmol)was added drop-wise (exothermic reaction). The resulting clear solution was allowed to stir atroom temperature for 30 min and was subsequently diluted with 100 mL CH2CI2. The organicphase was washed with 1 N HC1 (2 x 100 mL), dried on Na2SC>4 and concentrated to provide thecrude product (7.2 g, 88percent yield). *H NMR (400 MHz, CDC13) 8 1.71 (dd, J= 15.0, 7.2 Hz, 2H),2.12 (m, 2H), 2.38 (d, J= 15.9 Hz, 2H), 2.67 (m, 2H), 4.62 (s, 2H), 5.22 (s, 2H), 7.38 (m, 5H). |
| 86.4% |
With triethylamine; In dichloromethane; at 0 - 20℃; for 12h; |
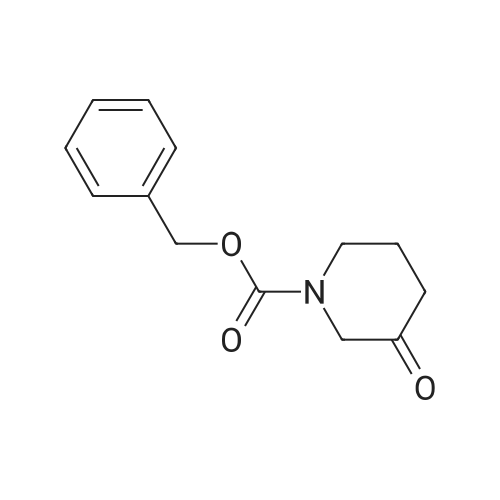
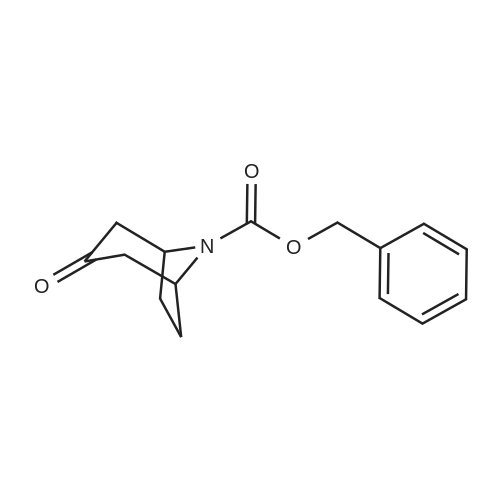
Norutoropinon hydrochloride (0.700g, 4.33mmol) in dichloromethane (20.0mL) solution of triethylamine (1.62mL, 11.7mmol) and benzyl chloroformate (0.733mL, 5.20mmol) at 0°C in addition, the mixture was stirred after raising the temperature 12 hours at room temperature. Distilled water was added to the reaction mixture, and the mixture was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate, filtered, and thefiltrate was concentrated under reduced pressure. The residue was purified by column chromatography (silica gel, nhexane/ ethyl acetate = 85 / 1555/ 45) to give benzyl 3-oxo-8-azabicyclo[3,2,1]octane-8-carboxylate (below to afford reference compound of example 12) (0.970g, 3.74mmol, 86.4percent) as a colorless oil. |
|
With triethylamine;dmap; In dichloromethane; at 0 - 20℃; |
A mixture of 8-Aza-bicyclo [3.2. 1] octan-3-one hydrochloride (2. g, 20mmol), TEA (4.2mL, 30mmol) and DMAP (cat. ) in DCM (60mL) was cooled to 0°C and benzyl CHLOROFORMATE (4.3mL, 30MMOL) was added and stirred for 30 minutes, then the reaction was allowed to stir at room temperature for overnight. The reaction was extracted with saturated ammonium chloride, brine and dried over magnesium sulfate. The organic layer was concentrated and purified on silica gel using heptane/ethyl acetate (7: 3) to give 2.62 grams of CBZ-protected 8-Aza-bicyclo [3.2. 1] octan-3-one. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping