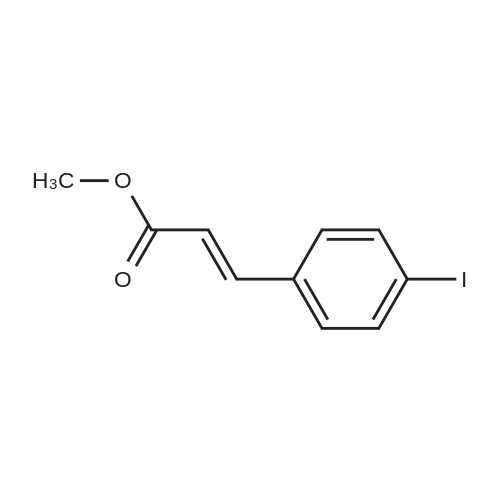
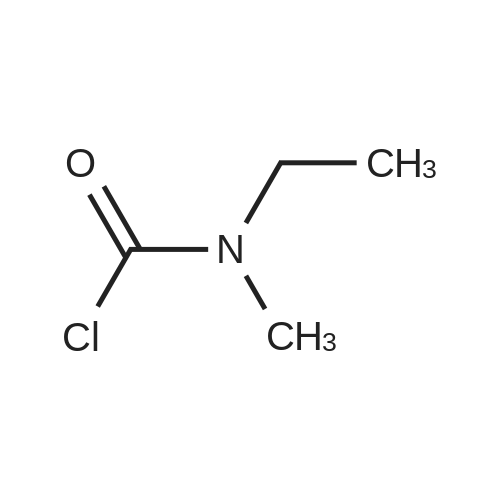
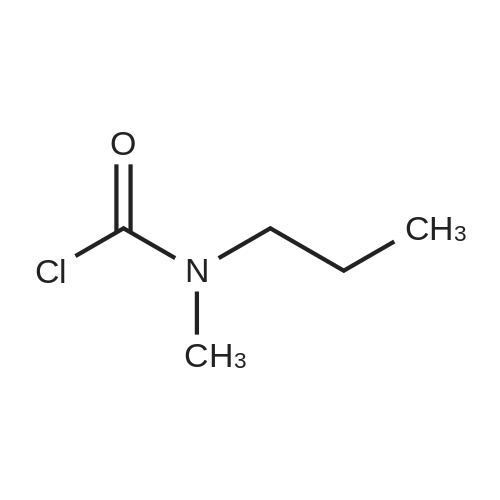
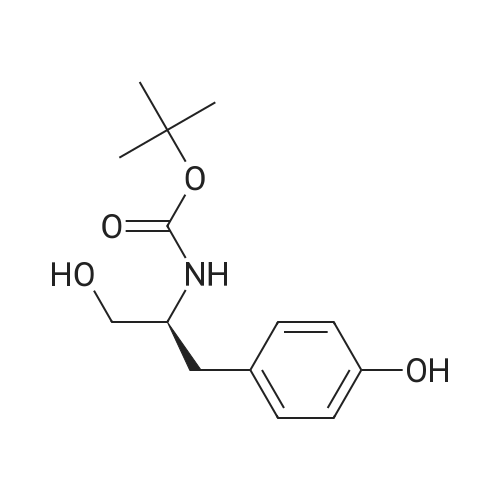
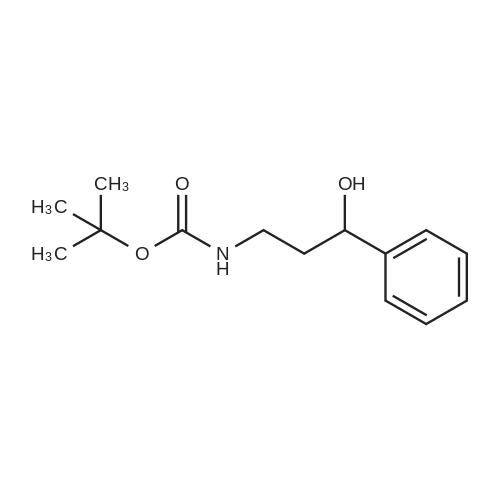
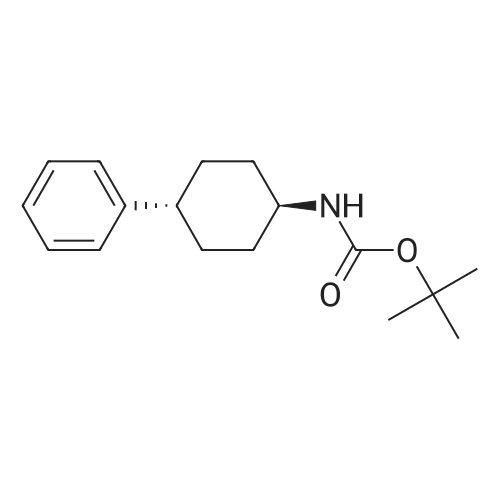
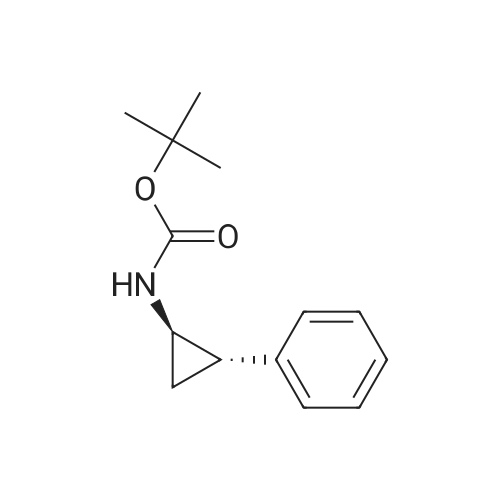
|
With triethylamine; In tetrahydrofuran; at 20℃;Cooling with ice; |
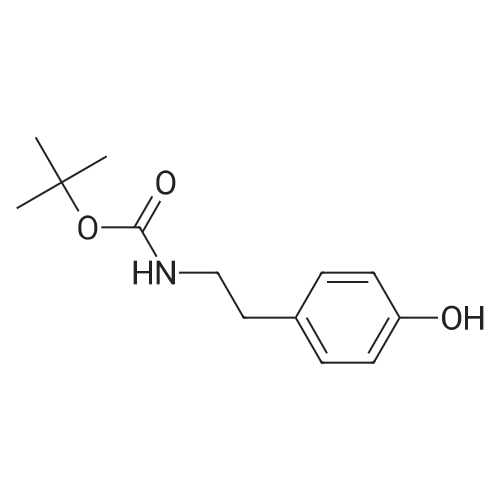
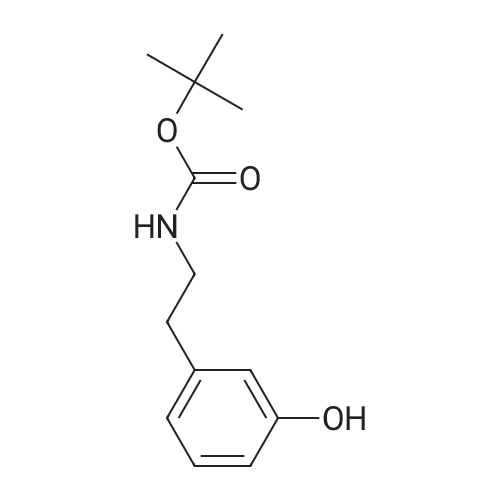
A solution of 2-(3-hydroxyphenyl)ethylamine (5.15 g, 29.7 mmol) in THF (65 mL) was treated with Et3N (9.30 mL, 2.2 equiv) and cooled on an ice-water bath. Di-tert-butyldicarbonate (6.80 g, 31.1 mmol) was added and the cooling bath was removed. After approx. 0.5 h THF (35 mL) and Et3N (5.0 mL) were added to improve solubility. After stirring overnight, the reaction mixture was diluted with EtOAc, washed with water and satd aq NaCl solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was dissolved in acetone (100 mL) and treated with benzyl bromide (3.90 mL, 33 mmol) and K2CO3 (4.50 g, 33 mmol). The reaction mixture was heated at 50 C for 19 h. The solvent was removed under reduced pressure. The residue was partitioned between CH2Cl2 and 2 M aq NaOH. The organic layer was washed with satd aq NaCl solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product, containing a small amount of residual benzyl bromide, was deprotected using general procedure 5 to give 2-(3-benzyloxyphenyl)ethylamine hydrochloride as a white solid, 5.49 g. |
|
With triethylamine; In tetrahydrofuran; for 0.5h;Cooling with ice; |
Cyclopenta[2,3]pyrrolo[2,1-a]isoquinoline-3-nitromethyl, 1,2,3,3a,4,5,7,8-octahydro-10-benzyloxy-2-methyl-5-oxo-(2S,3R,3aS,12bR) A solution of 2-(3-hydroxyphenyl)ethyl amine (5.15 g, 29.7 mmol) in THF (65 mL) was treated with Et3N (9.3 mL, 2.2 eq.) and cooled on an ice-water bath. Di-t-butyldicarbonate (6.8 g, 31.1 mmol) was added and the cooling bath was removed. After approx. 0.5 h, THF (35 mL) and Et3N (5 mL) were added to improve solubility. After stirring o.n., the reaction mixture was diluted with EtOAc, washed with water and sat. aq. NaCl solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was dissolved in acetone (100 mL) and treated with benzyl bromide (3.9 mL, 33 mmol) and K2CO3 (4.5 g, 33 mmol). The reaction mixture was heated at 50 C. for 19 h. The solvent was removed under reduced pressure. The residue was partitioned between dichloromethane and 2 M aq. NaOH. The organic layer was washed with sat. aq. NaCl solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product, containing a small amount of residual benzyl bromide, was deprotected by treatment with p-dioxane (20 mL) and 4 M HCl in dioxane (50 mL) at r.t. for 2 h. The reaction mixture was diluted with Et2O and the solid precipitate was filtered off, washed with Et2O and dried to give 2-(3-benzyloxyphenyl)ethyl amine hydrochloride as a white solid, 5.49 g. HPLC-(Method A)-tr=2.85 min. (94%). 1H-NMR (300 MHz, DMSO-d6): delta=8.04 (broad s, 3H, NH3+), 7.34-7.42 (m, 4H), 7.22 (t, J=7.7 Hz, 1H), 6.80-6.91 (m, 3H), 5.07 (s, 2H, PhCH2O), 3.00 (m, 2H), 2.81-2.85 (m, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping