| 66.7% |
With N-Bromosuccinimide; In acetonitrile; at 10 - 25℃; for 1h; |
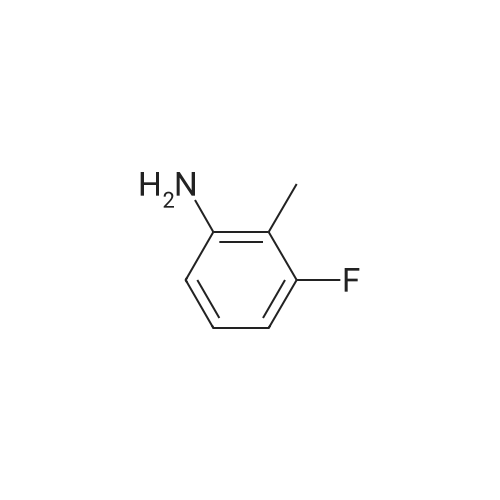
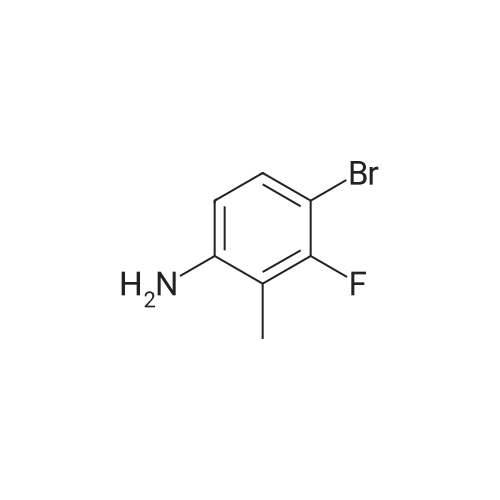
To a stirred solution of <strong>[443-86-7]3-fluoro-2-methylaniline</strong> (XXXI) (50 g, 399 mmol, 1.0 eq) in CH3CN (1.2 L) was added NBS (78 g, 439 mmol, 1.1 eq) in portions at 10°C, the resulting mixture was stirred at 25°C for 1 h. LC/MS showed the reaction was completed. Saturated Na2S203 (1.2 L) was then added slowly to the reaction mixture at 10°C, extracted with EtOAc (2 L) and the organic layer was concentrated under vacuum to give crude product. The residue was washed with PE (1 L), the solid was filtered, washed again with PE (500 mL) and dried under vacuum to give 4-bromo-<strong>[443-86-7]3-fluoro-2-methylaniline</strong> (XXXII) as a white solid (163.0 g, 798.9 mmol, 66.7percent yield). ESIMS found C7H7BrFN mlz 204.1 (M+l). |
| 66.7% |
With N-Bromosuccinimide; In acetonitrile; at 25℃; for 1h; |
To a stirred solution of <strong>[443-86-7]3-fluoro-2-methylaniline</strong> (XXXI) (50 g, 399 mmol, 1.0 eq) in CCN (1.2 L) was added NBS (78 g, 439 mmol, 1.1 eq) in portions at 10°C, the resulting mixture was stirred at 25°C for 1 h. LC/MS showed the reaction was completed. Saturated Na2S2C>3 ( 1.2 L) was then added slowly to the reaction mixture at 10°C, extracted with EtOAc (2 L) and the organic layer was concentrated under vacuum to give crude product. The residue was washed with PE (1 L), the solid was filtered, washed again with PE (500 mL) and dried under vacuum to give 4-bromo-<strong>[443-86-7]3-fluoro-2-methylaniline</strong> (XXXII) as a white solid (163.0 g, 798.9 mmol, 66.7percent yield). ESIMS found C7H7BrFN mlz 204.1 (M+l). |
| 66.7% |
With N-Bromosuccinimide; In acetonitrile; at 10 - 25℃; for 1h; |
To a stirred solution of <strong>[443-86-7]3-fluoro-2-methylaniline</strong> (XXIX) (50 g, 399 mmol, 1.0 eq) in CH3CN (1.2 L) was added NBS (78 g, 439 mmol, 1.1 eq) in portions at 10° C., the resulting mixture was stirred at 25° C. for 1 h. LC/MS showed the reaction was completed. Saturated Na2S2O3 (1.2 L) was then added slowly to the reaction mixture at 10° C., extracted with EtOAc (2 L) and the organic layer was concentrated under vacuum to give crude product. The residue was washed with PE (1 L), the solid was filtered, washed again with PE (500 mL) and dried under vacuum to give 4-bromo-<strong>[443-86-7]3-fluoro-2-methylaniline</strong> (XXX) as a white solid (163.0 g, 798.9 mmol, 66.7percent yield). ESIMS found C7H7BrFN m/z 204.1 (M+1). |
| 66.7% |
With N-Bromosuccinimide; In acetonitrile; at 10 - 25℃; for 1h; |
Step 1 (0819) To a stirred solution of <strong>[443-86-7]3-fluoro-2-methylaniline</strong> (XXIX) (50 g, 399 mmol, 1.0 eq) in CH3CN (1.2 L) was added NBS (78 g, 439 mmol, 1.1 eq) in portions at 10° C., the resulting mixture was stirred at 25° C. for 1 h. LC/MS showed the reaction was completed. Saturated Na2S2O3 (1.2 L) was then added slowly to the reaction mixture at 10° C., extracted with EtOAc (2 L) and the organic layer was concentrated under vacuum to give crude product. The residue was washed with PE (1 L), the solid was filtered, washed again with PE (500 mL) and dried under vacuum to give 4-bromo-<strong>[443-86-7]3-fluoro-2-methylaniline</strong> (XXX) as a white solid (163.0 g, 798.9 mmol, 66.7percent yield). ESIMS found C7H7BrFN m/z 204.1 (M+1). |
| 61% |
With N-Bromosuccinimide; In acetonitrile; at 10 - 20℃; for 3h; |
To a stirred solution of <strong>[443-86-7]3-fluoro-2-methylaniline</strong> (15.0 g, 120 mmol) in ACN (300.0 ml_) was added N-Bromosuccinimide (23 g, 132 mmol) portion wise at 10°C. The reaction mixture was stirred at ambient temperature for 3 h, and was evaporated under reduced pressure. The reaction mixture was diluted with saturated Na2S203 (100.0 ml_) at 10°C and extracted with EtOAc (2 X 100 ml_). Combined organic layer was washed with brine, dried over anhydrous Na2S04 and evaporated under reduced pressure to get desired crude, which was purified by column chromatography^ 00-200 mesh silica gel, eluent: 15percent ethyl acetate in hexane) of the title compound (15 g, 61percent) as a brown solid. LCMS rt 3.27 min MH+204. 1H NMR (400 MHz, DMSO-de) delta 7.09 (t, J=8.2, 1H), 6.40 (d, J=8.52, 1H), 1.98 (s, 3H) |
| 57% |
|
To a stirred solution of 3 -fluoro-2- methylaniline (25 g, 200 mmol) in acetic acid (140 mL) at 0-5 0C was added hydrogen bromide (100 mL, 200 mmol) then dimethyl sulfoxide (72 mL) was added slowly dropwise (reaction is exothermic and at temperature higher than 5-15 0C produces dibromoisomer). The mixture was stirred at 5-15 0C for 12 h (mixture became clear solution). The resulting solution was cooled to 0 0C and neutralized with sodium hydroxide then with sodium bicarbonate to pH 7. The mixture was extracted with ethyl acetate. The organic layer was concentrated under reduced pressure. Flash chromatography (0-10 percent ethyl acetate in hexane) gave the desired product as a white solid (23.3 g, 114 mmol, 57 percent yield). 1H NMR (400 MHz, CHLOROFORM-J) delta (ppm) 7.11 (t, J=8.20 Hz, IH), 6.35 (d, J=8.98 Hz, IH), 3.72 (br. s., 2H), 2.07 (d, J=I.95 Hz, 3H). |
| 46% |
With N-Bromosuccinimide; In acetonitrile; at 10 - 20℃; for 0.5h; |
To a stirred solution of <strong>[443-86-7]3-fluoro-2-methylaniline</strong> (2.0 g, 16.00 mmol, 1 equiv) in acetonitrile (50 ml_) was added N-bromosuccinamide (3.13 g, 17.60mmol, 1 .1 equiv) portionwise at 10°C. The resulting reaction mixture was stirred at room temperature for 30 min. Upon completion of the reaction, sat Na2S03 (50 ml_) was added slowly into the reaction mixture at 10°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic layers were dried over Na2S04 and concentrated in vacuo. The residue was triturated with n-pentane to affording the 4-bromo- <strong>[443-86-7]3-fluoro-2-methylaniline</strong> as light yellow solid which was used in the next step without further purification (1 .5g, 46percentyield).LC-MS (ES) m/z = 204.0, 206.0 [M+H]+. NMR (400 MHz, CDCI3) delta ppm 2.10 (s, 3 H), 6.39 (d, J = 8.4 Hz, 1 H), 7.13 (t, J = 8 Hz, 1 H), 7.60 -8.10 (br, 2 H). |
|
With N-Bromosuccinimide; In acetonitrile; at 10 - 20℃; for 0.5h; |
Example 9; Preparation of 5-Bromo-4-fluoro-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole (Intermediate 18)Step 1: 4-Bromo-<strong>[443-86-7]3-fluoro-2-methylaniline</strong> To a solution of <strong>[443-86-7]3-fluoro-2-methylaniline</strong> (20 g, 0.16 mol) in CH3CN (500 mL) was added NBS (31.3 g, 0.176 mol) in portions at 10° C. The resulting mixture was stirred at room temperature for 30 minutes. Upon completion, saturated Na2S2O3 (500 mL) was added slowly into the reaction mixture at 10° C. The organic layer was separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was washed with petroleum ether affording the title compound (20 g), which was used in the next step without further purification. 1H NMR (300 MHz, DMSO-d6): delta 7.08 (t, 1H), 6.40 (dd, 1H), 5.35 (br, 2H), 1.98 (d, 3H). |
|
With N-Bromosuccinimide; In acetonitrile; at 10 - 20℃; for 0.5h; |
To a solution of <strong>[443-86-7]3-fluoro-2-methylaniline</strong> (20 g, 0.16 mol) in CH3CN (500 mL) was added NBS (31.3 g, 0.176 mol) in portions at 10 °C. The resulting mixture was stirred at room temperature for 30 minutes. Upon completion, saturated Na2S2O3 (500 mL) was added slowly into the reaction mixture at 10 °C. The organic layer was separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was washed with petroleum ether affording the title compound (20 g), which was used in the next step without further purification. 1H NMR (300 MHz, DMSO-d6): oe 7.08 (t, 1H), 6.40 (dd, 1H), 5.35 (br, 2H), 1.98 (d, 3H). |
|
With N-Bromosuccinimide; In acetonitrile; at 0 - 10℃; for 0.5h; |
10292] To a stirred solution of <strong>[443-86-7]3-fluoro-2-methylaniline</strong> (15 g, 0.1199 mol) in acetonitrile (300 mE) was added N-bromo succinamide (23.5 g, 0.131 mol) at 0° C. The reaction mixture was stirred for 30 mm at 100 C., after completion of reaction (monitored by TEC), the reaction mixture was diluted with EtOAc. Organic layer was washed with water followed by brine, dried over anhydrous sodium sulphate, filtered and concentrated under reduced pressure. The crude product was used in next step without further purification to afford 4-bromo-<strong>[443-86-7]3-fluoro-2-methylaniline</strong> (25g, crude) as a white solid. |
|
With N-Bromosuccinimide; In acetonitrile; at 25℃; for 2h; |
Into a 250-mL a round bottle were placed <strong>[443-86-7]3-fluoro-2-methylbenzenamine</strong> (2.00 g, 15.982 mmol, 1.00 equiv.), NBS (2.99 g, 16.798 mmol, 1.051 equiv.), MeCN (100 mL). The resulting solution was stirred 2 h at 25° C. The reaction was then quenched by H2O. The resulting solution was extracted with EA and the organic layers combined and concentrated under vacuum. The residue was purified by silica gel column with PE:EA=70:30 to yield 4-bromo-<strong>[443-86-7]3-fluoro-2-methylbenzenamine</strong> as yellow solid. Mass spectrum (EI, m/z): Calculated for C7H7BrFN, 203.0 [M], found 202.9. |
|
With N-Bromosuccinimide; In acetonitrile; at 25℃; for 2h; |
Into a 250-mL a round bottle were placed <strong>[443-86-7]3-fluoro-2-methylbenzenamine</strong> (2.00 g, 15.982 mmol, 1.00 equiv.), NBS (2.99 g, 16.798 mmol, 1.051 equiv.), MeCN (100 mL). The resulting solution was stirred 2 h at 25° C. The reaction was then quenched by H2O. The resulting solution was extracted with EA and the organic layers combined and concentrated under vacuum. The residue was purified by silica gel column with PE:EA=70:30 to yield 4-bromo-<strong>[443-86-7]3-fluoro-2-methylbenzenamine</strong> as yellow solid. Mass spectrum (EI, m/z): Calculated for C7H7BrFN, 203.0 [M], found 202.9. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping