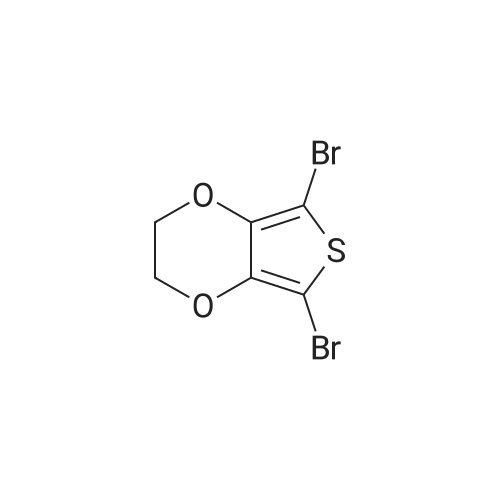
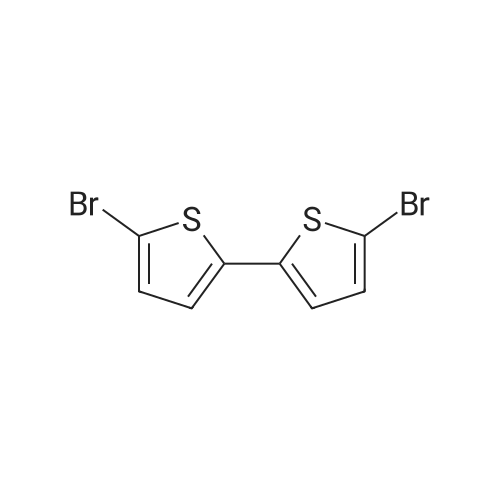
| 72% |
With N-Bromosuccinimide; for 1h; |
A mixture of bromosuccinimide (NBS) (2.75 g, 15.45 mmol) and N,N-dimethylformamide (DMF) (15 ml) was added dropwise to the mixture of EDOT (1.0 g, 7.03 mmol) and DMF (15 ml) and the reaction temperature was kept between 18-23 C. After completion of the addition, the mixture was stirred for 1 h, and then poured into an equal of ice water. After vigorous shaking for 5 min, the mixture was extracted with CH2Cl2, and washed with saturated NaHCO3 solution once and with water for three times. The combined organic layer was dried over anhydrous MgSO4 and concentrated under reduced pressure to leave a crude residue. Purification by recrystallization with n-hexane afforded 1.5 g 2,5-dibromo-3,4-ethylenedioxythiophene as a white solid in 72% yield. 1H NMR (400 MHz, CDCl3): delta 4.27 (s, 4H); 13C NMR: delta 139.7, 85.5, 64.9. |
| 33% |
With N-Bromosuccinimide; acetic acid; In tetrahydrofuran; at 20℃; for 3h; |
5-Br was synthesized based onliterature.5 3,4-Ethylenedioxythiophene (1.0 g, 7.04 mmol) and a mixture of THF/CH3COOH(24 mL, 1:1 v/v) were added to a dry 100 mL 2-neck round-bottom flask. NBS (3.13 g, 17.6 mmol)was added and the solution was stirred at room temperature for 3 h. Then, distilled water (50 mL)was poured to the reaction mixture. A silver crystalline solid was precipitated, filtered, dried andused without further purification (0.7 g, 33%). 1H NMR (300 MHz, DMSO-d6), delta: 4.30 (s, 4H).13C NMR (75 MHz, DMSO-d6), delta: 140.1, 84.2, 64.7. |
|
With N-Bromosuccinimide; In tetrahydrofuran; at 20℃; for 2h;Inert atmosphere; |
Compound 1 (0.3 g, 2.11 mmol) and N-bromosuccinimide (NBS)?(0.75 g, 4.22 mmol) were dissolved in 20 mL fleshly distilled THE and stirred at r.t. for 2 hr under N2. The reaction solution was transferred by syringe to a N2 protected 50 mL flask containing K2C03 (2 M, 5 ml), Pd(0)(PPh3)4 (0.129,0.1 mmol) and compound 4(1.47 g, 4.22 mmol). The reaction mixture was then stirred at reflux in the dark for 6 hr. The reaction mixture was cooled down to r.t. and poured into water, extracted with dichioromethane (DCM) and washed with water. The DCM layer was dried over MgSO4, concentrated and the residue mixture was purified by column chromatography on silica gel elutirig with DCM to obtain the product as a yellow solid (1.3 g, 82%). 1H NMR (CD2CI2) 6: 7.55 (br, 4H), 7.07 (d, J = 8.4 Hz, 8H), 6.90 (d, J = 7.6 Hz, 4H), 6.86 (d, J = 8.8 Hz, 12H), 4.34 (s, 4H), 3.81 (s, 12H). 13C NMR (CD2CI2) 6: 156.5, 147.7, 141.1, 138.3, 127.0, 125.8, 120.9, 115.1, 65.1, 55.9. HRMS (MALDI-TOF): calcd for C46H40N206S, 748.2607; found, 748.2656. Anal. calcd. for C46H40N206S: C, 73.78; H, 5.38; N, 3.74; S, 4.28 %. Found: C, 74.01; H, 5.29; N, 3.70; S, 4.21 %. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping