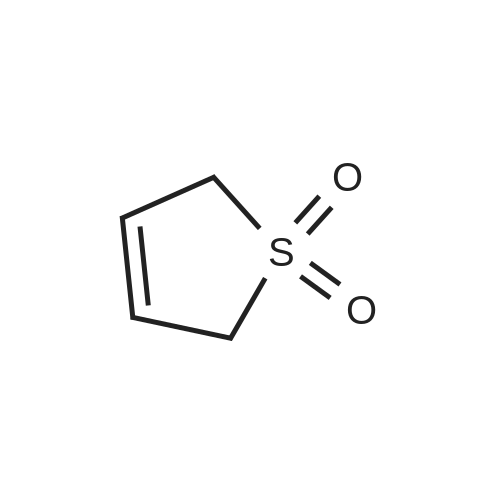
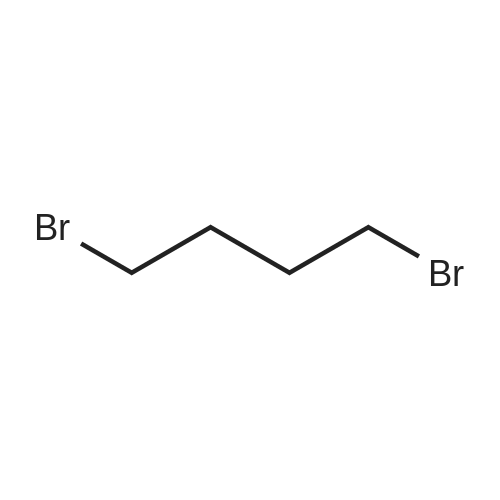
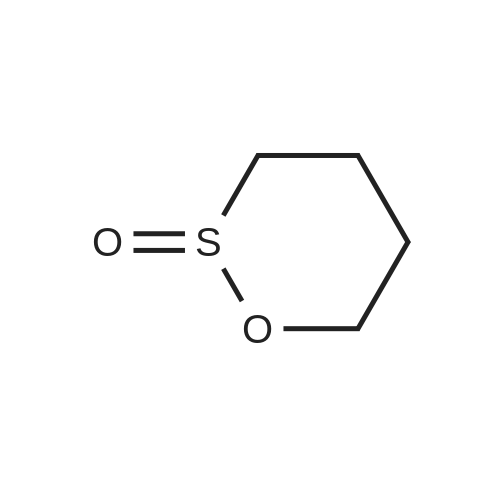
| 94% |
With (pyridinium)H3PMo11VO40; dihydrogen peroxide; In water; acetonitrile; at 40℃; for 2.5h; |
General procedure: The oxidation of methyl phenyl sulfide to methyl phenyl sulfoxide or methyl phenyl sulfone (Scheme 1) was typically carried out bystirring a solution of 0.7 mmol of the substrate and 0.01 mmol of the catalyst in 5 mL of acetonitrile, at 20 or 40 C, respectively. Theoxidant used was H2O2 35% in aqueous solution (2 or 20 mmol,respectively). The sample was collected from the reaction mixtureduring the reaction at time intervals. About 20 muL of the reactionmixture was taken for each sample, which was then diluted in amixture of water-dichloromethane (2 mL). The dichloromethanelayer was dried with anhydrous sodium sulfate and filtered. GC/MSanalyses were performed on an HP 5971 mass detector coupled to anHP gas chromatograph fitted with a 30 m×0.25 mm DB5 capillarycolumn. The percentages of each compound in the reaction mixturewere directly estimated from the corresponding chromatographicpeak areas. The yield (%) of pure sulfoxide or sulfone, the turnovernumber (TON: product mol×catalyst mol-1) and turnover frequency(TOF: product mol×catalyst mol-1×h-1) were also calculated. Under these optimum conditions and using M11PV1Py1 ascatalyst, different sulfides were oxidized to sulfoxides (for 30 min)and to sulfones (for 2.5 and 3.5 h) depending on the substrate. |
| 92% |
With 1,3,5-trichloro-2,4,6-triazine; dihydrogen peroxide; In water; acetonitrile; at 20℃; for 0.25h; |
General procedure: To a mixture of sulfide (1 mmol) and TCT (1 mmol, 0.184 g) in acetonitrile (5 mL) was added 30% H2O2 (2 mmol, 0.2 mL). The mixture was stirred at room temperature for the appropriate period of time until complete consumption of the starting material as observed by TLC. After completion of the reaction, H2O (10 mL) was added to the reaction mixture which was then extracted with EtOAc (4 × 5 mL) and the combined extracts were dried (MgSO4). The filtrate was evaporated and the corresponding sulfone was obtained as the only product (Table 1). |
| 92% |
With Octanoic acid; dihydrogen peroxide; In acetonitrile; at 50℃; for 0.416667h;Schlenk technique; Green chemistry; |
General procedure: An oven-dried Schlenk flask was allowed to cool toroom temperature and charged sequentially with sulfide(1.0 mmol), MeCN (3.0 mL) and caprylic acid (20 mol%).The reaction was then activated by the addition of 30%H2O2 (2.4 equiv.) and stirred at 50 C for the required timeas given in Table 4. The progress of reaction was monitoredby GC. After completion of the reaction, the reaction to the reaction mixture. Then the product was extractedwith CH2Cl2 (30 mL) and then washed with distilled water(10 mL). The organic extract dried over Na2SO4 and thesolvent removed under reduced pressure. The resultantproduct was purified (if necessary) by column chromatographyusing silica gel (60-120 mesh) with n-hexaneand ethyl acetate as solvent to get the pure product. Thestructure of the product was confirmed by GC-MS, M.P./B.P. and 1H NMR spectroscopic techniques. |
| 92% |
With 2,2,2-Trifluoroacetophenone; dihydrogen peroxide; acetonitrile; In tert-butyl alcohol; at 20℃; for 3h;pH 11;Green chemistry; |
General procedure: Sulfide (1.00 mmol) was placed in a round-bottom flask, followed by t-BuOH (0.5 mL), 2,2,2-trifluoroacetophenone (34.8 mg, 0.20 mmol), aq buffer solution (0.5 mL, 0.6 M K2CO3/4 × 10-4 M EDTA disodium salt), MeCN (0.15 mL, 3.00 mmol) and 30% aq H2O2 (0.36 mL, 3.00 mmol). The reaction mixture was stirred for 1-5 h. The reaction was quenched with 1 M HCl (5 mL) and extracted with CHCl3 (3 × 10 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo to afford the desired product. |
| 86% |
With anthracene; oxygen; acetic acid; In isopropyl alcohol; at 75℃; for 3h;Irradiation; |
General procedure: In the internal irradiation type photochemical reaction apparatus 4, 0.37 g (6 mmol) of dimethyl sulfide,Anthracene 0.21 g (1.2 mmol,20 mol% of dimethylsulfide) was dissolved in 75 mL of 2-propanol and 25 mL of acetic acid and stored.While supplying oxygen as Ultrafine bubbles at 5 C./minute at 75 C.,Light was irradiated by immersing the light source in the reaction solution.5 g of Amberlyst 15 (manufactured by Organo Corporation) was packed in the solid acid catalyst layer 6, placed in a circulation path before entering the reaction vessel,So that the organic acid in the reaction solution is brought into contact with the solid acid catalyst.The reaction solution was circulated for 2 hours while supplying Ultrafine bubble-form oxygen, and the raw material dimethylsulfide disappeared. The yield of dimethyl sulfone (DMSO 2) was analyzed by gas chromatography.The results are shown in Table 1. |
|
With tetrabutylammonium polychromiumphosphotungstate trihydrate; dihydrogen peroxide; In water; acetonitrile; at 25℃; for 0.166667h;Green chemistry; |
General procedure: PWCr catalyst (0.0245 mmol), CH3CN (3 mL), sulfide (1 mmol), and hydrogenperoxide (4 mmol, 30% aq solution) were added to a glass tube as the reaction vessel. The reaction was carried out at 298 K. The mixture was sampled periodically and analyzed by GC. After completion of the reaction, the product was extracted with CH2Cl2 and the combined organic layers were dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the corresponding pure sulfone. The products were identified by comparison of their 1H NMR and 13C NMR signals with the literature data (see Supplementary Data, Figures S5-S18). |
|
With dihydrogen peroxide; In water; acetonitrile; at 50℃; |
General procedure: A solution of sulfide (1 mmol) and catalyst (100 mg), in acetonitrile (9 mL), was added to H2O2 35% (w/v) (10 mmol). The mixture was stirred at 50 C for a time period (see Tables 1 and 2). The solvent was evaporated and then H2O (5 mL) was added. The substrate was extracted with toluene (2 × mL) and dried with anhydrous Na2SO4; filtration and evaporation afforded the corresponding sulfoxides. The crude solids were purified by recrystallization to affordthe pure sulfones |
|
With bis(N-tert-butylsalicylaldiminato)zinc(II); dihydrogen peroxide; In water; at 50℃; for 3.15h;Green chemistry; |
General procedure: To a mixture of the sulfide (1 mmol) and 30 % H2O2 (2 mmol), the catalyst (1 mmol) was added and the mixture was stirred at 50 C for a specified time. The progress of the reaction was monitored by TLC (petroleum ether/ethylacetate 8:3) and GC. After completion of the reaction, the product was extracted with ethyl acetate and the catalyst was separated by filtration. The combined organics were washed with brine (5 ml) and dried over anhydrous Na2SO4. Further purification was achieved by short-column chromatography on silica gel with EtOAc/n-hexane as the eluent. |
|
With bis(N-isopropylsalicylaldiminato)oxovanadium(IV); dihydrogen peroxide; In neat (no solvent); at 45℃; for 1.08333h;Green chemistry; |
General procedure: To a mixture of the sulfide (1mmol) and 30% H2O2 (1.5mmol), the catalyst (0.01mmol) was added and the mixture was stirred at 45C for a specified time. The progress of the reaction was monitored by TLC (petroleum ether/ethylacetate 8:3) and GC. After completion of the reaction, the product was extracted with ethyl acetate and the catalyst was separated by filtration. The combined organics were washed with brine (5ml) and dried over anhydrous Na2SO4. Further purification was achieved by short-column chromatography on silica gel with EtOAc/n-hexane as the eluent |
|
With C30H22Cl4N4O2Pd2; dihydrogen peroxide; In acetonitrile; at 50℃; for 4.15h; |
General procedure: A mixture of 1 mmol sulfide and H2O2 (3 mmol) wasadded to a stirring solution of CoL(NO3)2 (1), NiLCl2 (2),ZnL(NO3)2 (3) and Pd2LCl4 (4) Schiff base complexes(0.01 mmol) in acetonitrile (3 ml) at 50 C for the requiredperiod of time (4 h). After completion of the reaction (TLC),the catalyst was separated by filtration, washed three timeswith acetonitrile and then dried under vacuum and usedfor the next oxidation cycle. The products were analyzed byGC using diphenyl sulfide as the internal standard. |
|
With 4C16H36N(1+)*PW11CrO39(4-)*3H2O; dihydrogen peroxide; In water; at 25℃; for 0.166667h;Green chemistry; |
General procedure for the oxidation of sulfides to sulfones: The sulfide (1mmol) was added to a solution of 30% H2O2 (6.5 equiv) and TBAPWCr (16.5mumol), and the mixture was stirred at room temperature for the time specified in Table2. The progress of reactions was monitored by TLC and GC. After completion of the reaction, the product was extracted with ethyl acetate. Further purification was achieved by short-column chromatography on silica gel with EtOAc/n-hexane (1/10) as eluent. All of the products were known and characterized by 1HNMR and 13CNMR (see Supplementary data, Figs. S3-S15) [38-41]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping