|
With Dess-Martin periodane; In dichloromethane; at 20℃;Inert atmosphere; cooling with ice; |
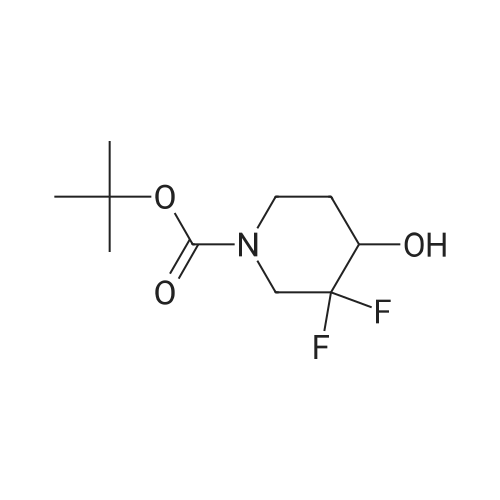
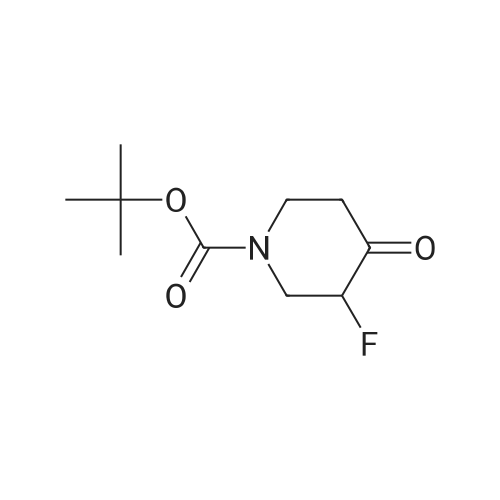
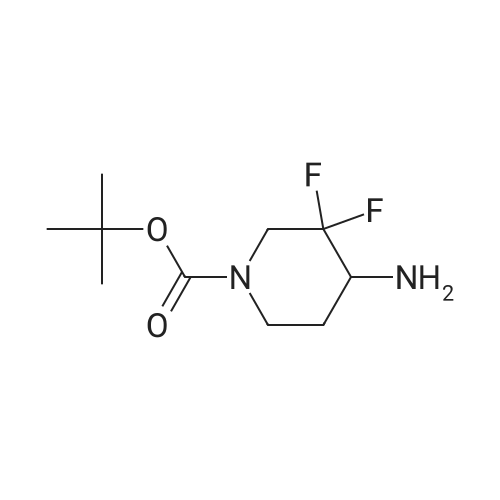
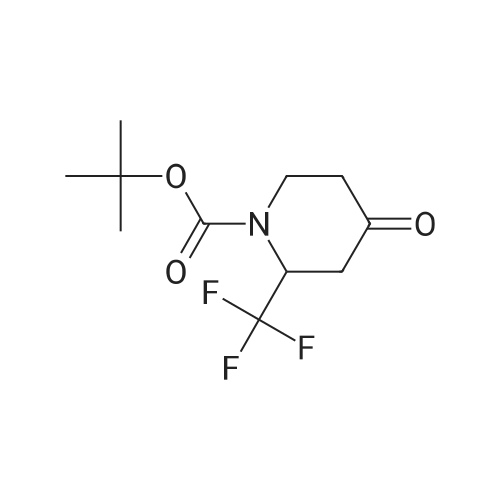
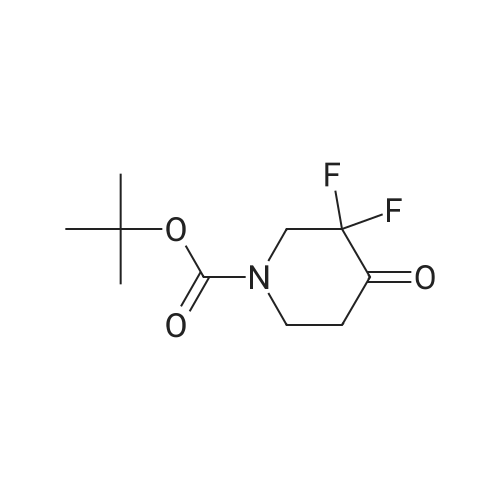
ferf-Butyl 4-(2-ethoxy-2-oxoethylidene)-3,3-difluoropiperidine-1 -carboxylate: In a flame dried round-bottomed flask equipped with a magnetic stir bar and under inert atmosphere (N2), to an ice-cold solution of ferf-butyl 3,3-difluoro-4-hydroxypiperidine-1- carboxylate (2.40 g, 10.00 mmol) in dry CH2CI2 (50 ml.) was added a solution of Dess- Martin periodinane (50 ml. of a 15% solution in CH2CI2, 24.00 mmol). The reaction mixture was stirred at rt until completion of the reaction. Sat. aq. NaHC03 (50 ml.) was then added followed by 10% aq. Na2S03 (50 ml_). The mixture was stirred at rt for 1 h, the layers separated and the aq. layer extracted with CH2CI2 (3x). The combined org. extracts were dried over MgS04, filtered, and concentrated under reduced pressure. The crude residue was redissolved in CH2CI2 (30 ml.) and stirred in the presence of molecular sieves for 24 h, filtered and concentrated under reduced pressure to give ferf-butyl 3,3-difluoro-4- oxopiperidine-1 -carboxylate as a yellow solid. In a flame dried round-bottomed flask equipped with a magnetic stir bar and under inert atmosphere (N2), to NaH (45 mg, 1 .12 mmol, 60% dispersion in oil washed with heptane) was added a solution of triethyl phosphonoacetate (262 mg, 1.17 mmol) in dry THF (10 ml.) at 0 C. The reaction mixture was stirred at 0 C for 30 min. Molecular sieves were then added followed by a solution of ferf-butyl 3,3-difluoro-4-oxopiperidine-1-carboxylate (220 mg, 0.93 mmol) in THF (5 ml_). The reaction mixture was stirred at 0 C for 30 min and at rt for 1 h. Water was then added and the mixture extracted with EA (3x). The combined org. extracts were dried over MgS04, filtered, and concentrated under reduced pressure to give the title compound as a yellow oil (mixture of E and Z isomers). LC-MS- conditions 08: tR = 0.88 and 0.93 min; [M-CH3+H]+ = 291.27. |
|
With Dess-Martin periodane; In dichloromethane; at 20℃;Inert atmosphere; |
tert-Butyl 4-(2-ethoxy-2-oxoethylidene)-3,3-difluoropiperidine-1-carboxylate In a flame dried round-bottomed flask equipped with a magnetic stir bar and under inert atmosphere (N2), to an ice-cold solution of <strong>[1209780-71-1]tert-butyl <strong>[1209780-71-1]3,3-difluoro-4-hydroxypiperidine-1-carboxylate</strong></strong> (2.40 g, 10.00 mmol) in dry CH2Cl2 (50 mL) was added a solution of Dess-Martin periodinane (50 mL of a 15% solution in CH2Cl2, 24.00 mmol). The reaction mixture was stirred at rt until completion of the reaction. Sat. aq. NaHCO3 (50 mL) was then added followed by 10% aq. Na2SO3 (50 mL). The mixture was stirred at rt for 1 h, the layers separated and the aq. layer extracted with CH2Cl2 (3*). The combined org. extracts were dried over MgSO4, filtered, and concentrated under reduced pressure. The crude residue was redissolved in CH2Cl2 (30 mL) and stirred in the presence of molecular sieves for 24 h, filtered and concentrated under reduced pressure to give tert-butyl 3,3-difluoro-4-oxopiperidine-1-carboxylate as a yellow solid. |
|
With Dess-Martin periodane; In dichloromethane; at 20℃;Inert atmosphere; |
In a flame dried round-bottomed flask equipped with a magnetic stir bar and under inert atmosphere (N2), to an ice-cold solution of terf-butyl 3,3-difluoro-4-hydroxypiperidine-1 - carboxylate (2.40 g, 10.00 mmol) in dry CH2CI2 (50 mL) was added a solution of Dess- Martin periodinane (50 mL of a 15% solution in CH2CI2, 24.00 mmol). The reaction mixture was stirred at rt until completion of the reaction. Sat. aq. NaHC03 (50 mL) was then added followed by 10% aq. Na2S03 (50 mL). The mixture was stirred at rt for 1 h, the layers separated and the aq. layer extracted with CH2CI2 (3x). The combined org. extracts were dried over MgS04, filtered, and concentrated under reduced pressure. The crude residue was redissolved in CH2CI2 (30 mL) and stirred in the presence of molecular sieves for 24 h, filtered, and concentrated under reduced pressure to give feri-butyl 3,3-difluoro-4- oxopiperidine-1 -carboxylate as a yellow solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping