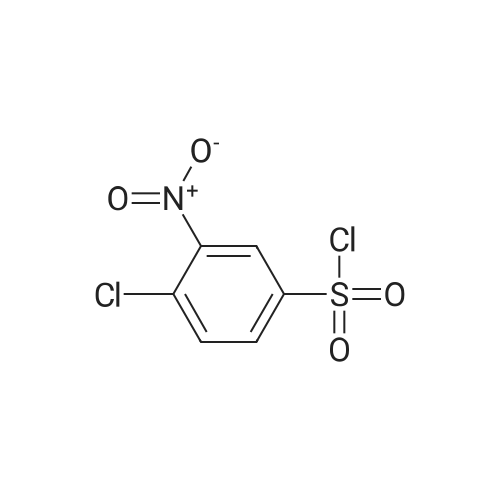
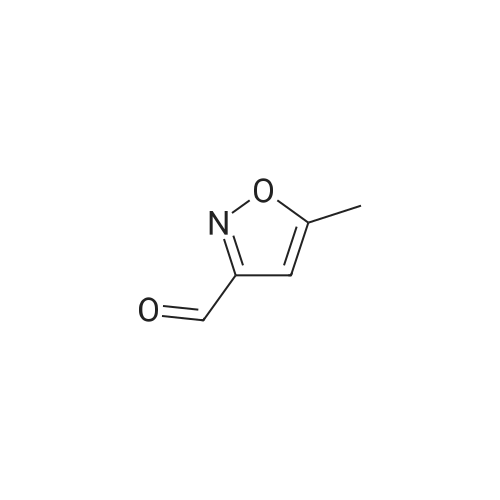
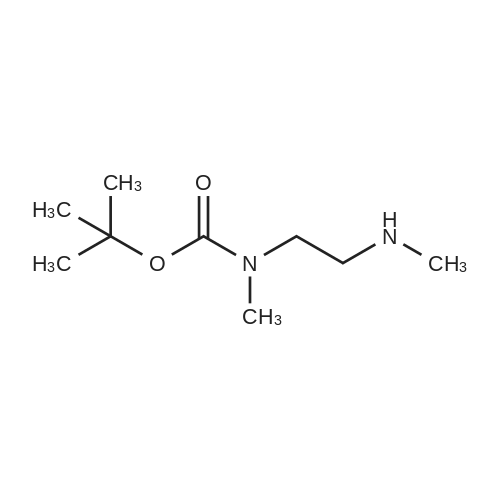
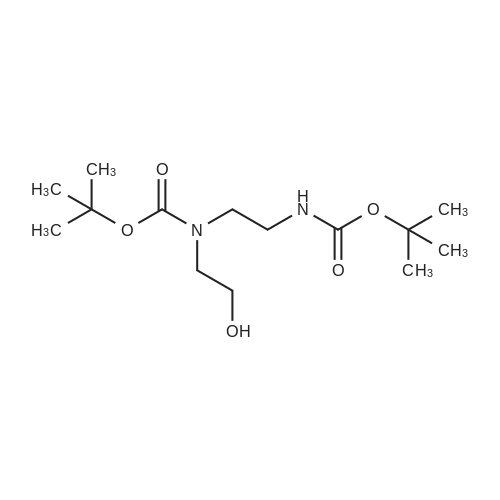
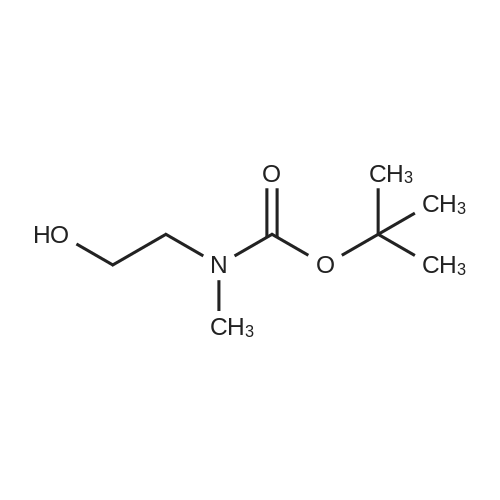
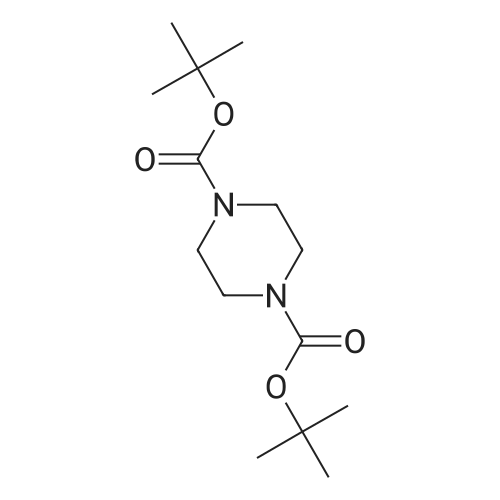
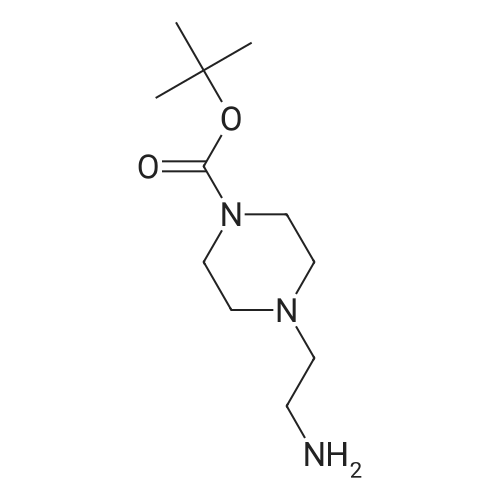
| 85% |
With N-ethyl-N,N-diisopropylamine; In isopropyl alcohol; at 90℃; for 5h; |
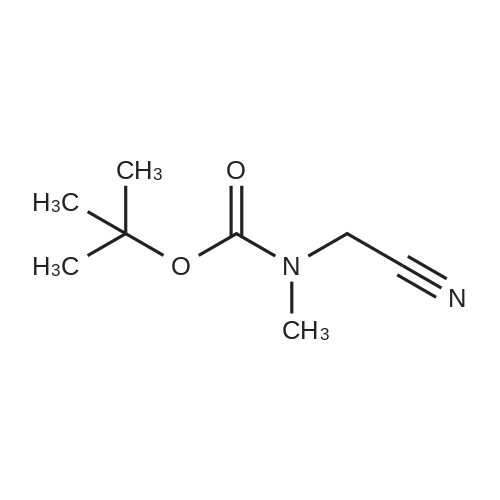
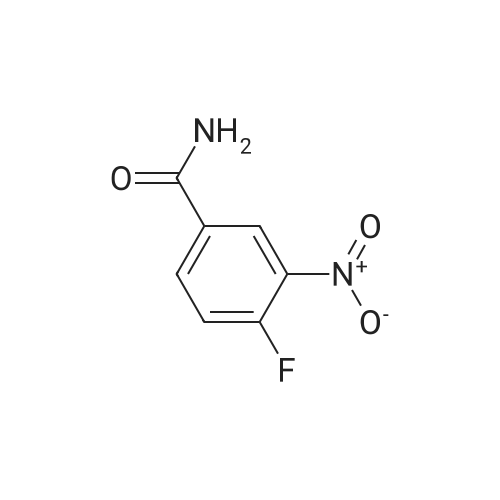
Di-tert-butyl dicarbonate (107 mg, 0.49 mmol) and triethylamine (0.07 ml, 0.49 mmol) were added to a solution of 2-bromoethanamine (100 mg, 0.49 mmol) in 1,4-dioxane at 0oC over 30 min. After stirring at rt for 48 h, precipitate was filtered off and the filtrate was concentrated under reduced pressure. The residue was taken by CH2Cl2and the resulting solution was washed with water, dried over MgSO4, and concentrated under reduced pressure. The crude product was purified by column chromatography (SiO2, Hexane:Acetone = 4:1) to givetert-butyl (2-bromoethyl)carbamate (100 mg, 0.45 mmol, 91% yield) as a colorless oil;1H NMR (400 MHz, (CD3)2CO) delta (ppm) 3.49 - 3.43 (m, 4H), 1.41(s, 9H).tert-Butyl (2-bromoethyl)carbamate obtained above (50 mg, 0.22mmol) was dissolved in DMF (1 ml). To this solution, NaN3(73 mg, 1.12 mmol) was added, and the mixture was stirred at 110oC for 12 h. Volatiles were removed under reduced pressure, and the residue was dissolved in water. The resulting aqueous solution was extracted with EtOAC. The organic layers were combined and dried over MgSO4. After filtration, the filtrate was concentrated under reduced pressure and the residue was purified by column chromatography (SiO2, Hexane:Acetone = 4:1) to givetert-butyl (2-azidoethyl)carbamate (40 mg, 0.21 mmol, 98% yield) as a colorless oil;1H NMR (400 MHz, CDCl3) delta (ppm) 3.41 - 3.40 (m, 2H), 3.32 - 3.29 (m, 2H), 1.45 (s, 9H).tert-Butyl (2-azidoethyl)carbamate (50 mg, 0.27 mmol) was dissolved in DMF (2 ml). To this solution, NaH (20 mg, 0.4 mmol) and MeI (0.03 ml, 0.4 mmol) were added at 0oC. After stirring for 5 h, the reaction mixture was treated with water followed by sodium thiosulfate and extracted with EtOAc. The organic layers were combined, dried over MgSO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, Hexane:Acetone = 4:1) to givetert-butyl (2-azidoethyl)(methyl)carbamate (40 mg, 0.20 mmol, 74% yield);1H NMR (400 MHz, CDCl3) delta (ppm) 3.38 (s, 4H), 2.90 (s, 3H), 1.45 (s, 9H).tert-Butyl (2-azidoethyl)(methyl)carbamate (180 mg, 0.9 mmol) obtained above was dissolved in THF. Water (0.1 ml) and triphenylphosphine (260 mg, 0.99 mmol) were added, and the mixture was stirred at rt for 10 h. After concentration under reduced pressure, the residue was purified by column chromatography (SiO2, Hexane:Acetone = 4:1 to CH2Cl2:MeOH:NH4OH:H2O=80:20:1:1) to givetert-butyl (2-aminoethyl)(methyl)carbamate (130 mg, 0.75 mmol, 83% yield);1H NMR (400 MHz, CDCl3) delta (ppm) 3.29 (br s, 2H) 2.92 - 2.89 (m, 5H), 1.46 (s, 9H).A solution of <strong>[349-02-0]4-fluoro-3-nitrobenzamide</strong> (238 mg, 1.3 mmol) ini-PrOH (5 ml) was treated with DIPEA (0.22 ml, 3.8 mmol) andtert-butyl (2-aminoethyl)(methyl)carbamate (450 mg, 3.8 mmol). After stirring for 5 h at 90oC, solvent was removed under reduced pressure to give a crude product which was purified by column chromatography (SiO2, CH2Cl2:MeOH:NH4OH:H2O = 80:20:1:1) to give5das a yellow powder (260 mg, 1.09 mmol, 85% yield);1H NMR (400 MHz, CD3OD) delta (ppm) 8.75 (s, 1H), 8.49 (br s, 1H), 7.98 (d,J= 7.2 Hz, 1H), 7.14 (d,J= 9.0 Hz, 1H), 3.52-3.48 (m, 2H), 3.37-3.34 (m, 2H), 3.31 (s, 3H), 1.42 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +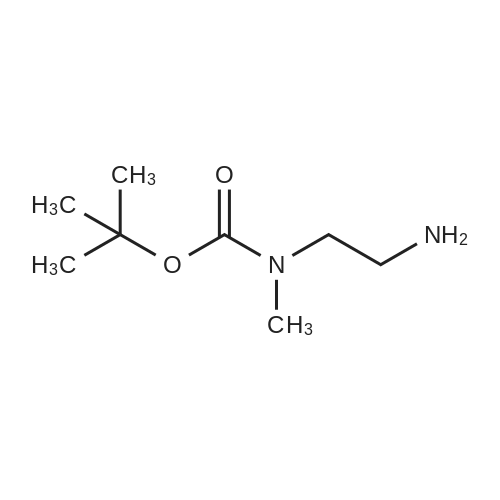

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping