|
With hydrogen;platinum on carbon; In methanol; at 20℃; under 1125.11 Torr; |
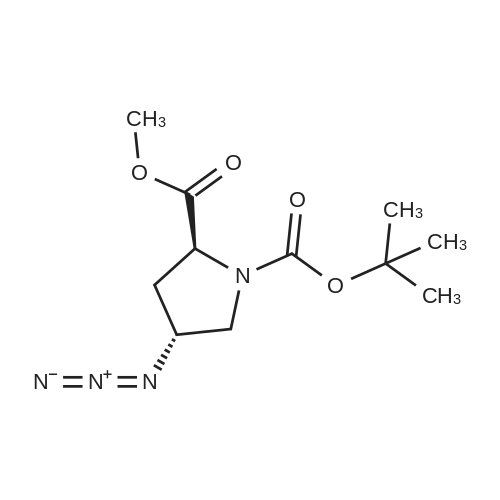
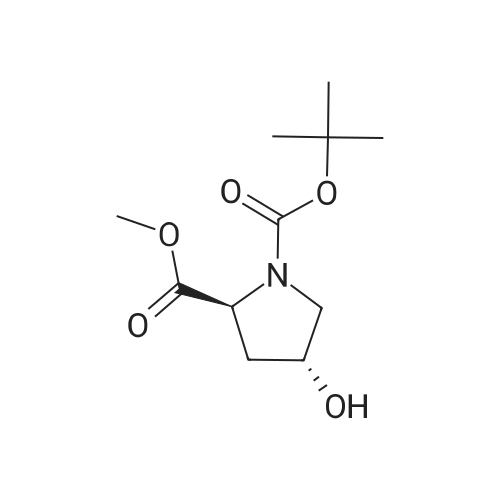
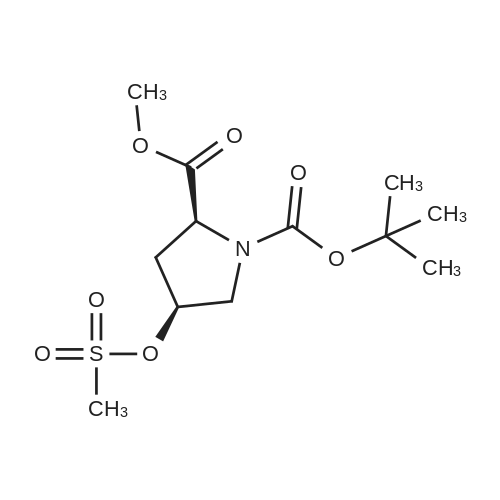
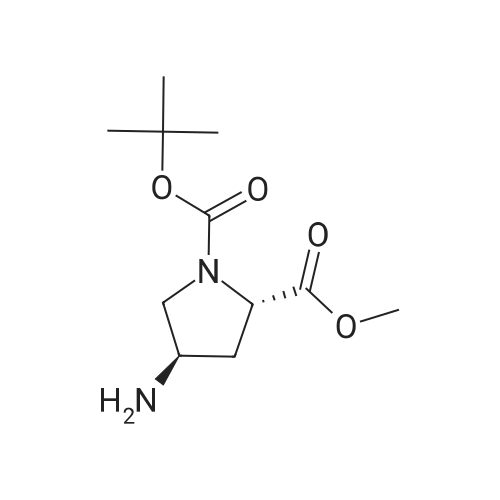
Example 4Into a pressure-proof reaction vessel of stainless steel (SUS) were placed 49.1 g (200 mmol, 1.00 eq) of optically active 4-hydroxyproline of the following formula (S-configuration at 2-position/R-configuration at 4-position, 98percent ee or higher, 98percent de or higher): 195 mL (1.03 M) of toluene, 200 mL (1.00 M) of acetonitrile, 40.5 g (400 mmol, 2.00 eq) of triethylamine and 133 g (413 mmol, 2.07 eq) of tetrabutylammonium bromide. Then, 40.8 g (400 mmol, 2.00 eq) of sulfuryl fluoride was blown from a cylinder into the reaction vessel under salt-ice cooling conditions. The resulting reaction mixture solution was stirred for 2 hours under salt-ice cooling conditions. It was confirmed by 1H-NMR analysis of the reaction mixture solution that the conversion rate of the reaction was 100percent.The thus-obtained reaction terminated liquid was washed with 300 mL of water. The organic layer was recovered, concentrated under a reduced pressure and subjected to vacuum drying, thereby yielding 145 g of a crude product of optically active 4-hydroxyl group substitution proline of the following formula (S-configuration at 2-position/S-configuration at 4-position): It was confirmed by 1H-NMR and 19F-NMR analysis of the crude product that no fluorinated compound of the following formula: was contained (less than 3 mol percent).It was also confirmed that there was a considerable amount of quaternary ammonium salt contained in the crude product (the mole ratio of the target compound and the quaternary ammonium salt was 53:47). To 68.4 g (estimated as 94.3 mmol) of the crude product, 51 mL (1.85 M) of toluene and 120 mL (0.786 M) of n-heptane were added. The resulting solution was stirred for 2 hours at room temperature, followed by filtering crystalline matter (quaternary ammonium salt) out of the solution. The filtration residue was washed with a small amount of n-heptane. The thus-obtained filtrate was concentrated under a reduced pressure and subjected to vacuum drying, thereby recovering 28.4 g of a purified product of optically active 4-hydroxyl group substitution proline of the above formula. It was confirmed by 1H-NMR analysis of the purified product that the quaternary ammonium salt had been totally removed (less than 3 mol percent). The yield of the product was 98percent. The 1H-NMR data of the optically active 4-hydroxyl group substitution proline was the same as that of Example 3.To 11.8 g (38.3 mmol, 1.00 eq) of the purified product of the optically active 4-hydroxyl group substitution proline of the above formula, 77 mL (0.497 M) of N,N-dimethylformamide and 2.74 g (42.1 mmol, 1.10 eq) of sodium azide were added. The resulting reaction mixture solution was stirred for 2 days at room temperature. It was confirmed by 1H-NMR analysis of the reaction mixture solution that the conversion rate of the reaction was 100percent.The thus-obtained reaction terminated liquid was diluted with 200 mL of ethyl acetate and washed three times with 100 mL of water. The organic layer was recovered, concentrated under a reduced pressure and subjected to vacuum drying, thereby yielding 12.5 g of a crude product of an azide compound of the following formula (S-configuration at 2-position/R-configuration at 4-position): It was confirmed by 1H-NMR analysis (quantitative analysis) of the crude product that 9.16 g of the target compound was contained (there was a considerable amount of N,N-dimethylformamide contained). The yield of the product was 89percent. The 1H-NMR data of the azide compound are indicated below. (No 4-position epimer (S-configuration at 4-position) was contained (less than 3 mol percent).)1H-NMR [reference material: (CH3)4Si, deuterium solvent: CDCl3] delta ppm; 1.42 (s, part of 9H), 1.47 (s, part of 9H), 2.18 (m, 1H), 2.33 (m, 1H), 3.42-3.84 (m, 2H), 3.74 (s, 3H), 4.20 (m, 1H), 4.38 (m, 1H).To 12.5 g (estimated as 33.9 mmol, 1.00 eq) of the crude product of the azide compound of the above formula, 34 mL (0.997 M) of methanol and 2.89 g (50percent water content, 0.679 mmol, 0.02 eq) of 5percent palladium/activated carbon were added. The resulting reaction mixture solution was stirred for one night at room temperature while setting the pressure of hydrogen gas (H2) at 0.15 MPa. It was confirmed by 1H-NMR analysis of the reaction mixture solution that the conversion rate of the reaction was 100percent.The thus-obtained reaction terminated liquid was subjected to Celite filtration. The filtrate was admixed with 3.53 g (33.9 mmol, 1.00 eq) of 35percent hydrochloric acid, stirred for 15 minutes at room temperature, concentrated under a reduced pressure, subjected to azeotropic dehydration twice with 100 mL of toluene, and then, subjected to vacuum drying. With this, 11.1 g of a crude product of hydrochloride of amino compound of the following formula (S-configuration at 2-position/R-configuration at 4-position): was yielded (There was contained a small amount of N,N-dimethylformamide and toluene). The yield o... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping