| 96.4% |
|
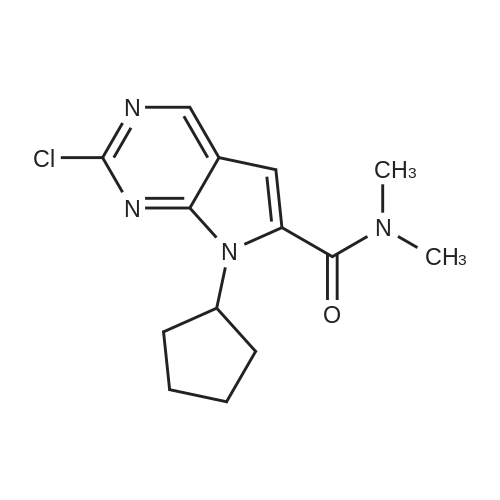
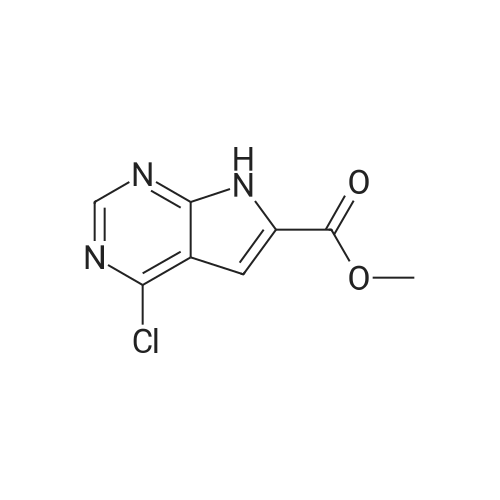
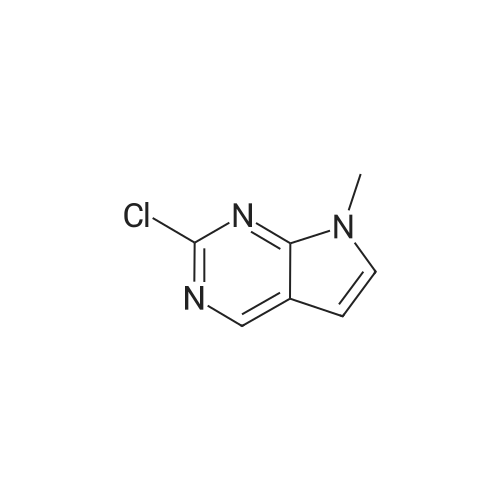
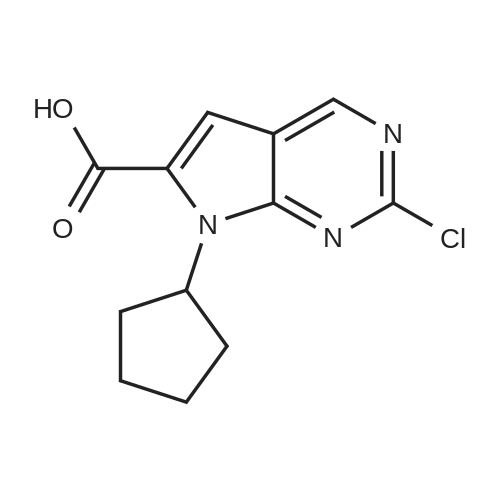
Toluene (500 mL) and 2-Chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidine- 6-carboxylic acid (lOO.Og, 0.377 moles) were charged into 2L 4N RB flask under CaCb guard tube condition, and stirred of 5-10 min to get cream colored suspension. Added N,N-dimethylformamide (10.0 mL) and stirred for 5-10 min. Charged thionyl chloride (67.2g, 0.566 moles) to the reaction mass and stir for 5-l0min. at 25-35C to get pale brown colored suspension. Reaction mass was heated to 75-80C and stirred for 3-4h. After completion of reaction (by HPLC), cooled to 0-5C to become brown colored suspension. Mixture of 2M solution of dimethylamine in THF (470.0 mL, 0.942 moles) and triethylamine (l22.0g, 1.20 moles) added dropwise to the reaction mass through addition funnel by maintaining the mass temperature at 0-10C and then stirred for 5-10 min. Reaction mass temperature was raised to 25-35 C and stirred for 1 -2h. After completion of reaction (by HPLC) solvent was distilled off under vacuum on rotavapor at 60-65 C to yield brown colored crude. The resulting crude was dispersed in DM water (1000.0 mL) and stirred for lh at 25-35C to get cream colored suspension. Product was filtered under suction with the help of DM water (500 mL). The wet product was dried in hot air oven at 60-65 C for 6h to afford the title compound. Wt. of the product l06.0g (96.4% by theory). Purity by HPLC > 99.0%. |
| 92% |
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine; In tetrahydrofuran; N,N-dimethyl-formamide; at 0 - 25℃; |
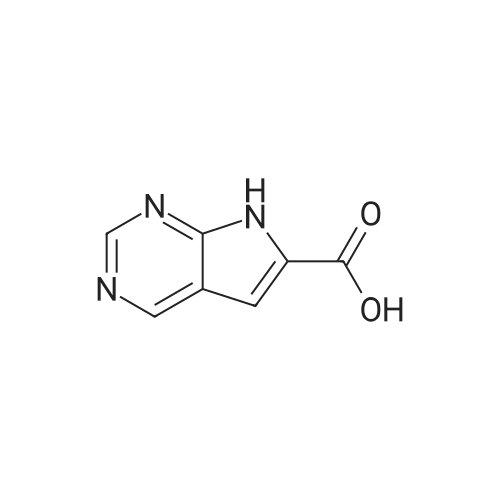
A three-necked flask was charged with 5 (26.57 g, 100 mmol)And N, N-dimethylformamide (133 mL) were added, the mixture was cooled to 0-5 C while stirring, EDCI (23.00 g, 120 mmol) was added,A solution of dimethylamine in tetrahydrofuran (2.0 M, 75 mL, 150 mmol) was added. Triethylamine (20.24 g, 200 mmol) was added dropwise and the mixture was stirred at 20-25 C for 6-8 hours.The reaction mixture was extracted with ethyl acetate (133 mL) three times and the combined organic phases washed twice with brine (133 mL), dried over anhydrous sodium sulfate and concentrated. Compound A was isolated by column chromatography on a mixture of dichloromethane and methanol (26.93 g , 92%). |
| 92% |
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine; In tetrahydrofuran; N,N-dimethyl-formamide; at 0 - 25℃; |
<strong>[1211443-58-1]2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid</strong> (26.57 g, 100 mmol) and N,N-dimethylformamide (133 mL) were added to a three-neck flask. stir well, then cool to 0 ~ 5 C, add EDCI (23.00g, 120mmol), A solution of dimethylamine tetrahydrofuran (2.0 M, 75 mL, 150 mmol) was added, and triethylamine (20.24 g, 200 mmol) was added dropwise, and the mixture was reacted at 20 to 25 C for 6 to 8 hours. The reaction was extracted with ethyl acetate (133 mL) three times.The organic phase was washed twice with saturated brine (133 mL).After concentration, it was beaten with isopropyl alcohol and water, and the solid was separated by filtration. drying in vacuo to gave 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide (26.93 g, 92%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping