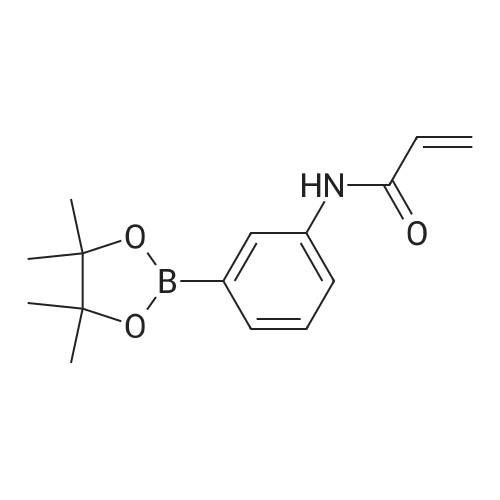
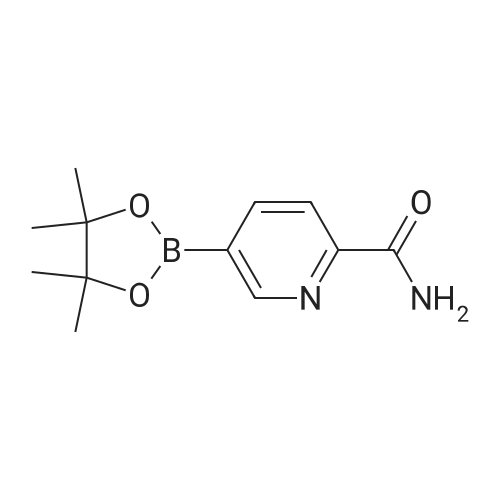
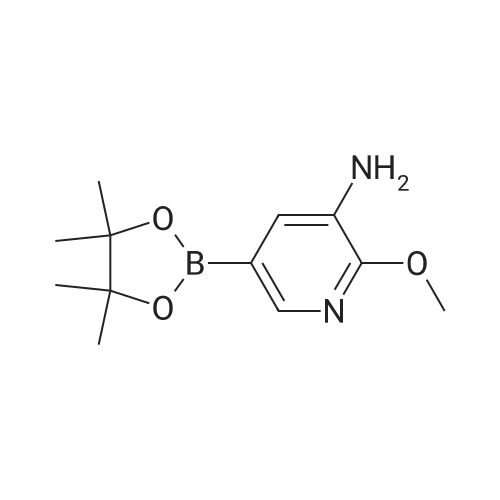
| 92.1% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 100.0℃;Inert atmosphere; Industrial scale; |
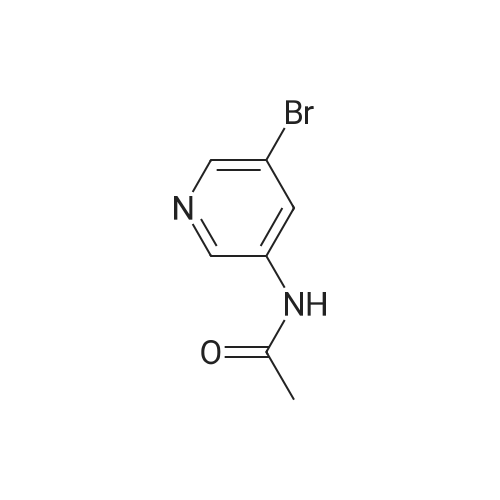
A 1 L four-necked flask equipped with a magnetic stirrer, a thermometer, a reflux condenser and a bubbler was charged(0.23 mol) of 3-acetylamino-5-bromopyridine, 58.42 g (0.23 mol) of bis (pinacolato) diboron and 67.62 g (0.69 mol) of potassium acetate were added and 450 mL of dioxane was added. Under protection, 3.80 g (0.0051 mol) of ferrocenepalladium chloride was added, and the reaction was heated to 100 C. for 18-24 hours,TLC control to the end of the reaction, the temperature was precipitated solid, beating filtration, methanol was added 500mL dissolved, filtered and evaporated to dryness, add heptane beating, to give the product 55.40g, yield 92.1%. |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 100.0℃; for 1.0h;Sealed tube; Microwave irradiation; |
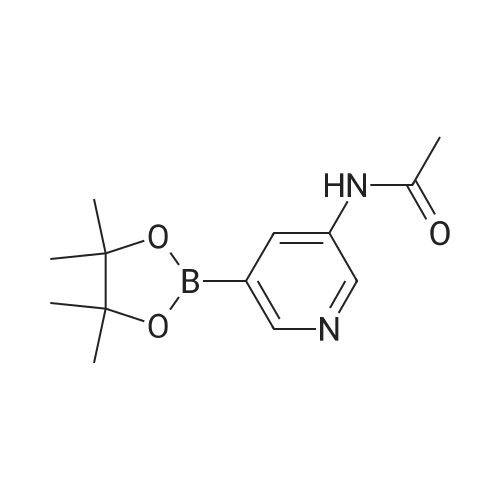
Intermediate 61 : N-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-yl)acetamide To a suspension of A/-(5-bromopyridin-3-yl)acetamide (for a preparation see Intermediate 60, 563 mg, 2.62 mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(1 ,3,2-dioxaborolane) (1330 mg, 5.24 mmol) and potassium acetate (771 mg, 7.85 mmol) in 1 ,4-dioxane (8 mL) in a microwave vial, was added PdC idppf) (192 mg, 0.262 mmol). The vial was sealed and the mixture was heated in a microwave at 100C for 1 h. The mixture was dissolved in ethyl acetate and filtered through a Celite cartridge (10 g). The solvent was evaporated under reduced pressure to give A/-(5-(4,4,5,5-tetramethyl-1 ,3,2- dioxaborolan-2-yl)pyridin-3-yl)acetamide as a brown oil (1.77 g, 258 %). This crude material was used in the next step without further purification: 100 % conversion assumed therefore maximum purity of crude material is 39 %. LCMS (2 min, High pH): Rt = 0.47 min, MH+ 263 |
|
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,4-dioxane; at 85.0℃; for 8.0h;Inert atmosphere; |
To a 25 mL round-bottom flask was charged with N-(5-bromopyridin-3-yl)acetamide (500 mg, leq), Bis(pinacolato)diboron (l. leq), PdCl2(dppf) (0.05eq), AcOK (3eq) in 15 mL of dioxane. The mixture was thoroughly degassed by alternately connecting the flask to vacuum and nitrogen. The solution was heated at 85 0C for 8 h. The solvent was removed in vacauo to afford a mixture containing N-(5-(4,4,5,5-tetramethyl -l,3,2-dioxaborolan-2-yl)pyridin-3-yl)acetamide. To the mixture, compound 43 (leq . 2 M K2CO3 (5 eq) and Pd(PPh3)4 (10 mg) and DMF (1OmL) was added. The reaction mixture was stirred at 155 0C for 8h under N2 protection. The mixture was diluted with water (10 mL) and extracted with DCM (3 X 20 mL). Organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by column chromatography to give compound 44 (377 mg, 65%(two step)). 394. 1H-NMR: (delta, ppm, CDC13, 400MHz): 10.33 (s, IH), 9.50 (s, IH), 8.86 (s, IH), 8.78-8.75 (m, 3H), 8.45 (s, IH), 8.24-8.22 (d, IH), 7.94-7.91 (d, IH), 7.32-7.27 (m, 5H), 5.92 (s, 2H), 2.11 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping