| 70% |
With racemic-2-(di-tert-butylphosphino)-1,1?-binaphthyl; zinc;palladium(II) trifluoroacetate; In ISOPROPYLAMIDE; at 50 - 95℃;Inert atmosphere; |
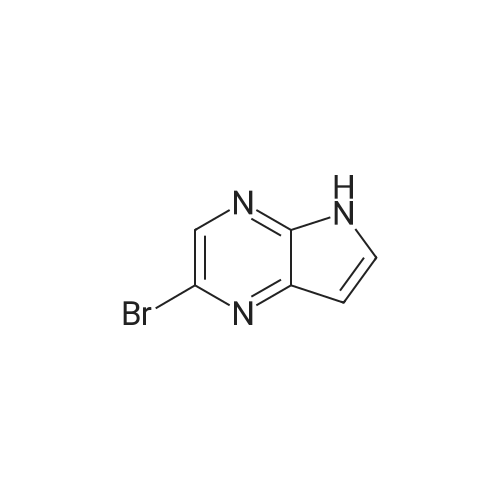
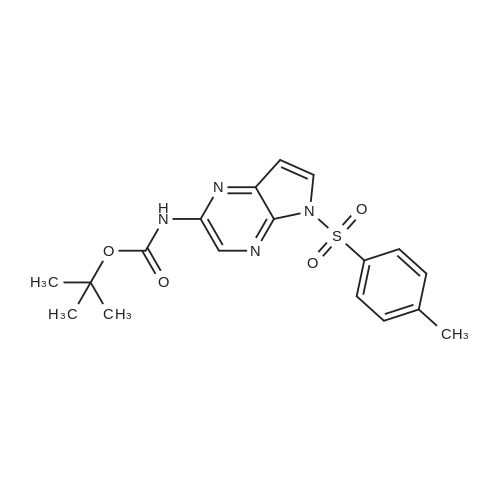
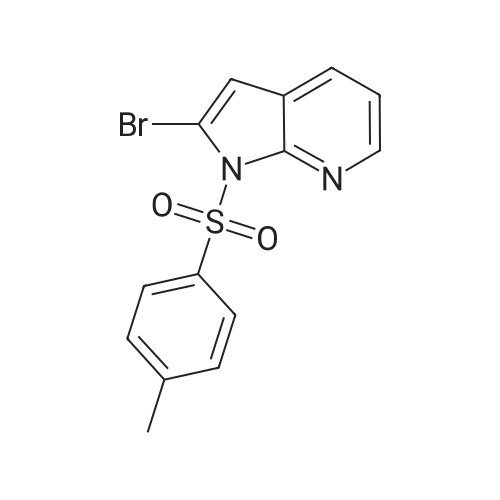
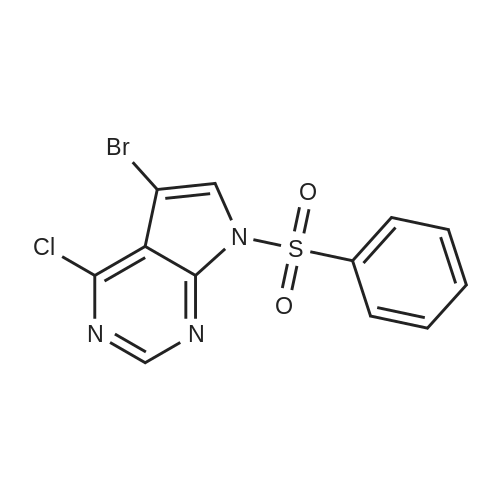
A 5-L reactor was charged with <strong>[1201186-54-0]2-bromo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine</strong> (98.8 g, 281 mmol, Preparation No.7), zinc dust (3.50 g, 53.3 mmol), palladium (II) trifluoroacetate (4.0 g, 12 mmol), and racemic-2-(di-t-butylphosphino)-1,1'-binapthyl (9.8 g, 24.7 mmol). The flask was equipped with a powder addition device into which zinc cyanide (10.0 g, 157 mmol) was placed to be added at a later step. The vessel was purged with argon for no longer than about 30 min and then argon sparged DMA (2 L) was added to the reactor. The mixture was stirred and heated to about 50 C. while maintaining an argon sparge. The resulting dark brown solution was further heated to about 95 C. while adding the zinc cyanide, from the powder addition device, portion-wise over about 15 min. Upon reaching about 95 C., the brown mixture is stirred for about an additional 16 h. The reaction mixture was cooled to room temperature, resulting in the precipitation of salts. The mixture was filtered through a Buchner funnel containing filter-aid and the filter cake was washed with DMA (20 mL). A solution of the crude product in DMA was added to cold (<10 C.) water (16 L) and stirred for about 30 min. The resulting suspension was filtered and the filter cake was rinsed again with water (1 L). The resulting wet cake was dried in a vacuum oven at about 50 C. The crude solid was dissolved in DCM (1.5 L) and further dried over anhydrous MgSO4. After filtration, the solution was passed through a pad of silica (140 g), washing with additional solvent until only predominantly impurities were detected eluting off the pad. The solvent was removed and the crude solid was triturated with MeOH/DCM (4:1, 10 volumes of solvent per gram of crude solid) at ambient temperature for about 5 h. The solid was filtered and washed with MeOH (300 mL). The product was dried in a vacuum oven to provide 5-tosyl-5H-pyrrolo[2,3-b]pyrazine-2-carbonitrile (58.8 g, 70%) as a colorless solid: 1H NMR (400 MHz, CDCl3) delta 8.67 (s, 1H), 8.21 (d, J=4.2 Hz, 1H), 8.07 (d, J=8.4 Hz, 2H), 7.34 (d, J=8.1 Hz, 2H), 6.89 (d, J=4.2 Hz, 1H), 2.42 (s, 3H). A 2-L 316-stainless steel pressure reactor was charged with 5% Pd/C (15.4 g of 63.6 wt % water wet material, 5.6 g dry basis, Johnson Matthey A503032-5), 5-tosyl-5H-pyrrolo[2,3-b]pyrazine-2-carbonitrile (55 g, 184 mmol), THF (1.1 L), deionized water (165 mL), aqueous HCl, (37 wt %, 30 mL, 369 mmol) and quinoline (1.1 mL, 9.0 mmol). The vessel was purged, pressurized, and maintained at 40 psi with hydrogen supplied from a high pressure reservoir. The mixture was vigorously agitated at about 25 C. After about 5 h the reactor was vented and purged with nitrogen to remove most of the dissolved hydrogen, and the reaction mixture was filtered to remove the catalyst. The reactor and catalyst cake were rinsed with THF:H2O (1:1, 2×40 mL). The combined filtrate and rinses were concentrated and EtOH (500 mL) was added. After two additional solvent switches with EtOH (2×500 mL), the crude residue was concentrated to give a residue (76 g) that was suspended in EtOH (550 mL) and stirred at ambient temperature for about 4 h. The solid was collected by filtration and washed with cold EtOH (50 mL). The wet cake was dried in a vacuum oven to provide (5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-yl)methanamine hydrochloride (51.2 g, 82%) as a colorless solid: LC/MS (Table 2, Method a) Rt=1.44 min; MS m/z: 303 (M+H)+. |
| 58.8% |
With tetrakis(triphenylphosphine) palladium(0); In N,N-dimethyl-formamide; at 110℃;Inert atmosphere; |
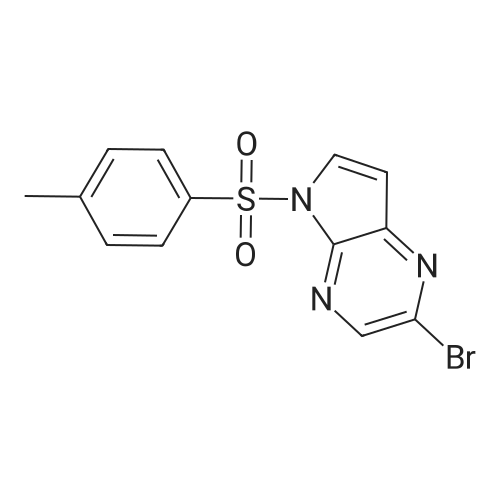
The compound <strong>[1201186-54-0]2-bromo-5-p-toluenesulfonyl-5H-pyrrolo[2,3-b]pyrazine</strong> 5b (4.0 g, 11.3 mmol), Zinc cyanide (2.4 g, 22.7 mmol) and Pd(PPh3)4 (1.6 g) were dissolved in DMF (100 ml) and reacted at 110 C overnight under nitrogen. TLC showed completion of the reaction, 150ml of ice water was added, extracted with ethyl acetate (100 mL) the aqueous phase twice, the organic phases are combined, once, dried over anhydrous sodium sulfate washed with brine, and dried to give the crude product under reduced pressure using a rotary evaporator, through the column to give the title compound 5c (2.0g, 6.7mmol), a yield of 58.8% |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping