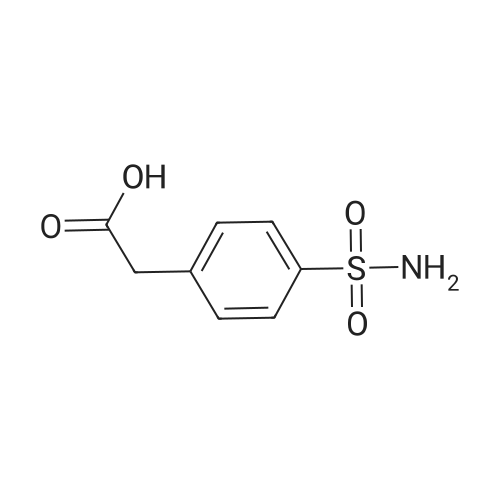
Alternatived Products of [ 119018-29-0 ]
Product Details of [ 119018-29-0 ]
| CAS No. : | 119018-29-0 |
MDL No. : | MFCD02955393 |
| Formula : |
C16H21N3O4S
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | AJEMFZRCUKJSES-UHFFFAOYSA-N |
| M.W : |
351.42
|
Pubchem ID : | 11772520 |
| Synonyms : |
|
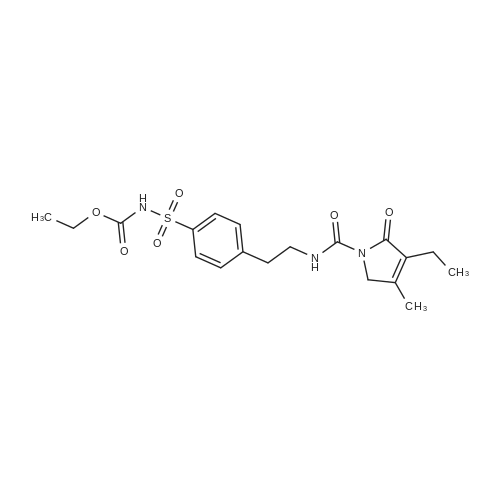
Chemical Name : | 4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide |
Safety of [ 119018-29-0 ]
Application In Synthesis of [ 119018-29-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 119018-29-0 ]
- 1
-
 [ 599174-27-3 ]
[ 599174-27-3 ]

-
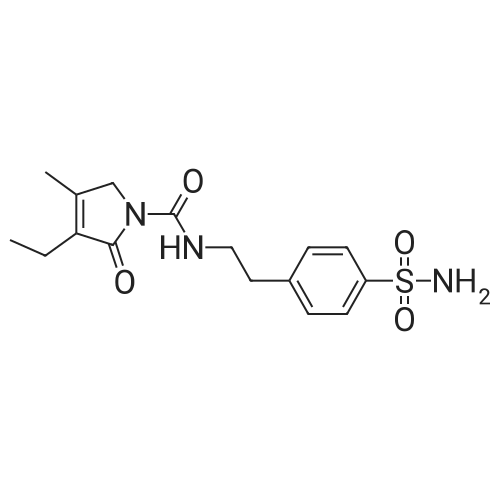 [ 119018-29-0 ]
[ 119018-29-0 ]

-
 [ 599174-28-4 ]
[ 599174-28-4 ]
- 2
-
 [ 119043-16-2 ]
[ 119043-16-2 ]

-
 [ 119018-29-0 ]
[ 119018-29-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 97.8% |
With ammonia; In water; at 80℃; for 1.5h; |
120 g of chlorosulfonic acid was added to a three-necked bottle, and the compound m was added in portions. After the addition, the reaction was kept at 80 C for 0.5 hour. The reaction was completely monitored by TLC, and the temperature was lowered to 25 C. The reaction was dropped into ice water and filtered to obtain a solid. Adding solid to a solution of 200 ml of ammonia water and 100 ml of water, heating to 80 C for 1.5 hours, cooling to 30 C, adding hydrochloric acid to the reaction solution, separating the solid, filtering, washing with water, drying at 50 C, The obtained white solid was 25.2 g, and the purity by HPLC was 78.08%, the impurity V 7.24%, the impurity was 16.35%, and the yield was 97.8%. |
|
With ammonia; at 70℃; for 3 - 4h; |
Example 2Preparation of 4-[2-(3-Ethyl-4-methyl-2-carbonyl pyrrolidine amido) ethyl ] benzene sulfonamide (IV)3-Ethyl-4-methyl-2,5-dihydro-lH- pyrrole-2-one (II) (1.0 Kg) and beta-phenylethyl isocyanate (1.488 Kg) were mixed in anhydrous toluene (4.0 L) and refluxed for 4 hrs. The toluene was distilled off and hexane (8.0 L) was added to the reaction mixture at 5O0C. The product precipitated is cooled to 0 to 5 C to obtain the solid compound viz. 4-[2-(3-Ethyl-4-methyl-2-carbonyl pyrrolidine amido) ethyl] benzene (2.17 Kg). It was filtered & washed with 2.0 L of hexane.To a cooled (15 to 25 C) solution of chlorosulfonic acid (2.8 L), 4-[2-(3-Ethyl-4- methyl-2-carbonyl pyrrolidine amido) ethyl] benzene (2.0 Kg) was added in small portions over a period of 2 to 3 hrs. Further it was stirred for 30 min at this temperature and then temperature was gradually raised to 30 to 350C. The reaction mass is stirred further for 2 hrs. The reaction mixture was then quenched into ice- water and stirred for 1 hr and filtered to obtain the product 4-[2-(3-Ethyl-4-methyl-2- carbonyl pyrrolidine amido) ethyl] benzene sulfonyl chloride (2.0 kg). To a cooled (15 to 200C) solution of diluted ammonia (1.4 L) 4-[2-(3-Ethyl-4-methyl-2-carbonyl pyrrolidine amido) ethyl] benzene sulfonyl chloride was added in small portion over 1 to 2 hrs. The reaction mixture was then heated to 7O0C for 2 hrs when ammonolysis is complete. The product converted is then stirred for 1 hr at R.T. and filtered and dried at 90. to 100 C to obtain crude 4-[2-(3-Ethyl-4-methyl-2-carbonyl pyrrolidine amido) ethyl] benzene sulfonamide (2.2 Kg) having HPLC purity in the range of 82 to 88%. The crude compound 4-[2-(3-Ethyl-4-methyl-2-carbonyl pyrrolidine amido) ethyl] benzene sulfonamide (2.2 Kg) is then purified from mixture of organic solvents chosen from Methanol, Acetone & toluene. |
| 8.47 kg |
With ammonium hydroxide; In methanol; at 20 - 30℃; for 2h;Industrial scale; |
38.0 kg of methanol was sequentially added to a 100 L reactor.Industrial ammonia water 5.1Kg; start stirring,Add the above intermediate 2 crude wet product at 20-30 C,And the reaction was kept for 2 hours; the sample was controlled by HPLC, and the reaction was completely filtered and washed with water.The obtained white intermediate 3 crude wet product was directly charged into another 200 L reaction kettle.Further, 110.0 kg of methanol was added, and the temperature was raised to 65-75 C, and the mixture was stirred for 4 hours.Cool down to 20-25 C, filter,Drying white solid intermediate 3 refined product 8.47kg, HPLC purity >99%,The meta isomer impurity was 0.2 <%, and the molar yield was 64% (based on the intermediate 1). |
Reference:
[1]Patent: CN110092739,2019,A .Location in patent: Paragraph 0022
[2]Tetrahedron Letters,2003,vol. 44,p. 4853 - 4855
[3]Patent: WO2006/103690,2006,A1 .Location in patent: Page/Page column 7,14-15
[4]Patent: CN108383768,2018,A .Location in patent: Paragraph 0101; 0102
- 3
-
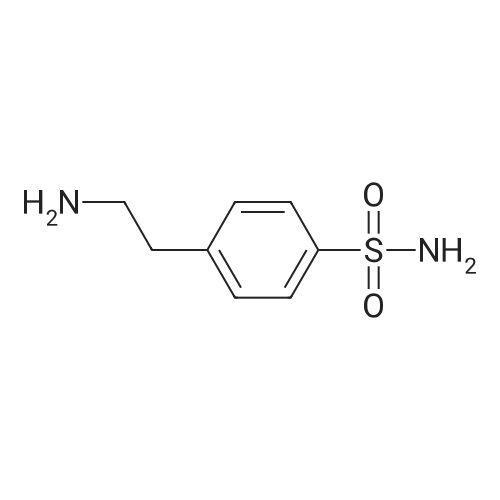
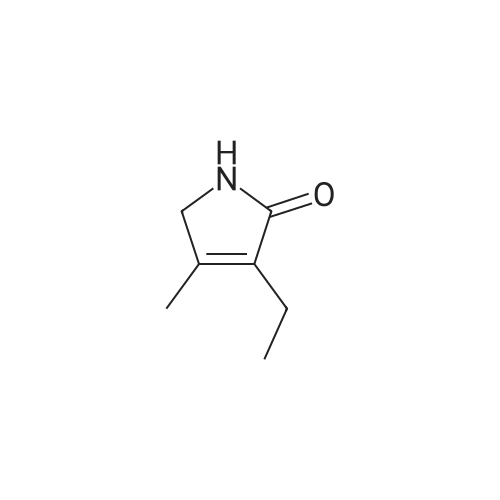 [ 766-36-9 ]
[ 766-36-9 ]

-
 [ 119018-29-0 ]
[ 119018-29-0 ]
- 4
-
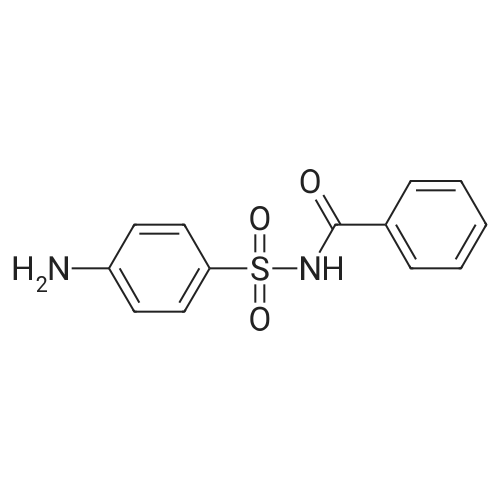
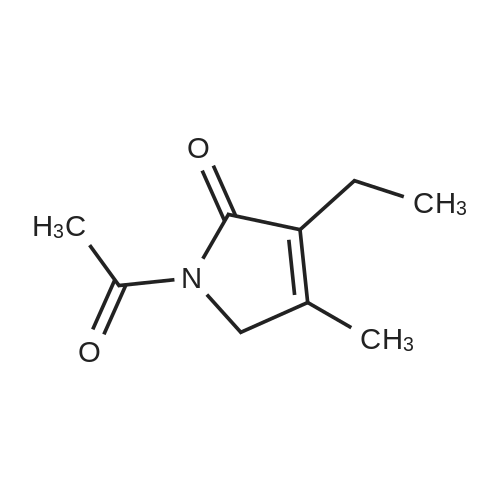 [ 61892-80-6 ]
[ 61892-80-6 ]

-
 [ 119018-29-0 ]
[ 119018-29-0 ]
- 5
-
 [ 247098-17-5 ]
[ 247098-17-5 ]

-
 [ 119018-29-0 ]
[ 119018-29-0 ]
- 6
-
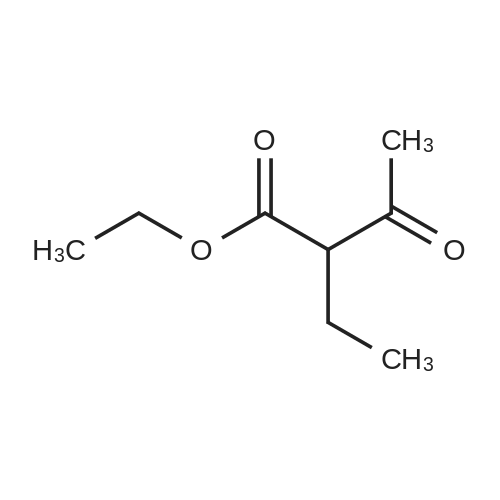 [ 607-97-6 ]
[ 607-97-6 ]

-
 [ 119018-29-0 ]
[ 119018-29-0 ]
- 7
-
 [ 247098-18-6 ]
[ 247098-18-6 ]

-
 [ 119018-29-0 ]
[ 119018-29-0 ]
- 8
-
 [ 119018-29-0 ]
[ 119018-29-0 ]

-
trans-hydroxyglimepiride
[ No CAS ]
- 9
-
 [ 119018-29-0 ]
[ 119018-29-0 ]

-
cis-hydroxyglimepiride
[ No CAS ]
- 10
-
 [ 541-41-3 ]
[ 541-41-3 ]

-
 [ 119018-29-0 ]
[ 119018-29-0 ]

-
C19H25N3O6S
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 83% |
With triethylamine; In dichloromethane; at 5 - 30℃; |
Examples: Example 1: Preparation of carbamate of Formula IX (R'= ethyl, and Grp1=D) In a reaction vessel 100 gm of 4[2-(3-ethyl-4-methyl-2-carbonyl pyrrolidine amido) ethyl] benzene sulfonamide, 72 gm triethyl amine and 1.6 lit dichloromethane were taken and the mixture cooled to 0 C. 46.15 gm of ethyl chloroformate was diluted separately with 200 ml dichloromethane and added into the reaction vessel drop wise while maintaining the temperature at around 5 C and maintained for 2 hours at 5 C and then at 25 to 30 C till completion of reaction. 2 L water and 0.7 L dichloromethane was added and the pH of the reaction mass were adjusted to 4 by addition of acetic acid. The organic layer was separated and washed with water and concentrated to dryness. The residue was refluxed with 300 ml acetone and cooled to 25 to 30 C, maintained for 1 hour, filtered and washed with 100 ml chilled acetone to get 100 gm carbamate of Formula IX (yield 83percent, purity 99.7percent). Melting Point: 177 to 182 C |
| 83% |
With triethylamine; In dichloromethane; at 0 - 30℃; for 2h; |
In a reaction vessel 100 g of 4[2-(3-ethyl-4-methyl-2-carbonyl pyrrolidine amido) ethyl] benzene sulfonamide, 72 g triethyl amine, and 1.6 L of dichloromethane were mixed and cooled to 0° C. 46.15 g of ethyl chloroformate was diluted separately with 200 mL of dichloromethane and added into the reaction vessel drop wise while maintaining the temperature at around 5° C. for 2 hours, and then at 25 to 30° C. until completion of the reaction. 2 L of water and 0.7 L of dichloromethane were added; and the pH of the reaction mass was adjusted to 4 by addition of acetic acid. The organic layer was then separated, washed with water, and concentrated to dryness. The residue was refluxed with 300 mL of acetone and cooled to 25 to 30° C., maintained for 1 hour, filtered, and washed with 100 mL of chilled acetone to obtain 100 g carbamate of Formula IX (yield 83percent, purity 99.7percent, and melting point: 177 to 182° C.). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping