| 51% |
|
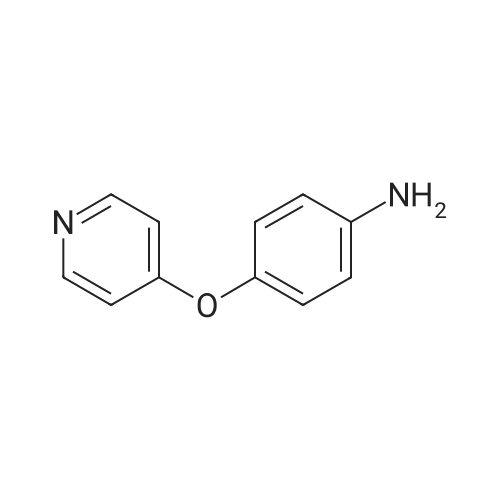
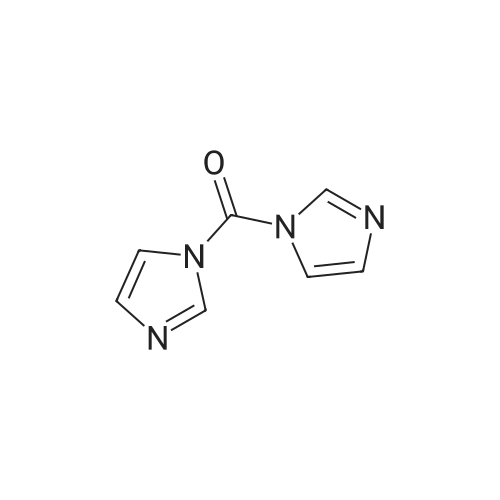
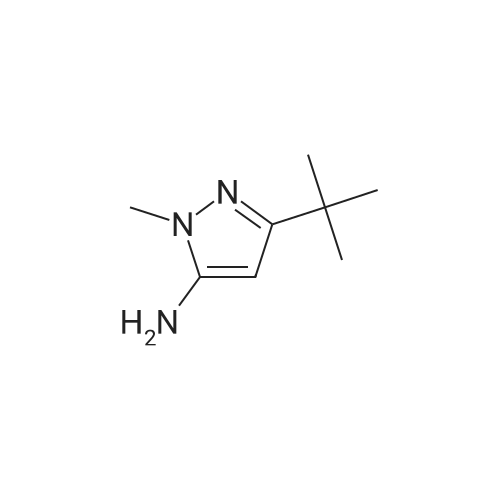
C3a. Reaction of a Heterocyclic Amine with N,N'-Carbonyldiimidazole Followed by Reaction with a Substituted Aniline; N-(3-tert-Butyl-1-methyl-5-pyrazolyl)-N'-(4-(4-pyridinyloxy)phenyl)urea; To a solution of 5-amino-3-tert-butyl-1-methylpyrazole (189 g, 1.24 mol) in anh. CH2Cl2 (2.3 L) was added N,N'-carbonyldiimidazole (214 g, 1.32 mol) in one portion. The mixture was allowed to stir at ambient temperature for 5 h before adding <strong>[102877-78-1]4-(4-pyridinyloxy)aniline</strong>. The reaction mixture was heated to 36 C. for 16 h. The resulting mixture was cooled to room temp, diluted with EtOAc (2 L) and washed with H2O (8 L) and a saturated NaCl solution (4 L). The organic layer was dried (Na2SO4) and concentrated in vacuo. The residue was purified by crystallization (44.4% EtOAc/44.4% Et2O/11.2% hexane, 2.5 L) to afford the desired urea as a white solid (230 g, 51%): mp 149-152 C.; 1H-NMR (DMSO-d6) delta 1.18 (s, 9H), 3.57 (s, 3H), 6.02 (s, 1H), 6.85 (d, J=6.0 Hz, 2H), 7.08 (d, J=9.0 Hz, 2H), 7.52 (d, J=9.0 Hz, 2H), 8.40 (d, J=6.0 Hz, 2H), 8.46 (s, 1H), 8.97 (s, 1H); FAB-LSIMS m/z 366 ((M+H)+). |
| 51% |
|
To a solution of 5-amino-3-tert-butyl-1-methylpyrazole (189 g, 1.24 mol) in anh. CH2Cl2 (2.3 L) was added N,N'-carbonyldiimidazole (214 g, 1.32 mol) in one portion. The mixture was allowed to stir at ambient temperature for 5 h before adding <strong>[102877-78-1]4-(4-pyridinyloxy)aniline</strong>. The reaction mixture was heated to 36 C for 16 h. The resulting mixture was cooled to room temp, diluted with EtOAc (2 L) and washed with H2O (8 L) and a saturated NaCl solution (4 L). The organic layer was dried (Na2SO4) and concentrated in vacuo. The residue was purified by crystallization (44.4% EtOAc/44.4% Et2O/11.2% hexane, 2.5 L) to afford the desired urea as a white solid (230 g, 51%): mp 149-152 C; 1H-NMR (DMSO-d6) delta 1.18 (s, 9H), 3.57 (s, 3H), 6.02 (s, 1H), 6.85 (d, J=6.0 Hz, 2H), 7.08 (d, J=9.0 Hz, 2H), 7.52 (d, J=9.0 Hz, 2H), 8.40 (d, J=6.0 Hz, 2H), 8.46 (s, 1H), 8.97 (s, 1H); FAB-LSIMS m/z 366 ((M+H)+). |
|
|
N-(3-tert-Butyl-1-methyl-5-pyrazolyl)-N'-(4-(4-pyridinyloxy)phenyl)urea: To a solution of 5-amino-3-tert-butyl-1-methylpyrazole (189 g, 1.24 mol) in anh. CH2Cl2 (2.3 L) was added N,N'-carbonyldiimidazole (214 g, 1.32 mol) in one portion. The mixture was allowed to stir at ambient temperature for 5 h before adding <strong>[102877-78-1]4-(4-pyridinyloxy)aniline</strong>. The reaction mixture was heated to 36 C. for 16 h. The resulting mixture was cooled to room temp, diluted with EtOAc (2 L) and washed with H2O (8 L) and a saturated NaCl solution (4 L). The organic layer was dried (Na2SO4) and concentrated in vacuo. The residue was purified by crystallization (44.4% EtOAc/44.4% Et2O/11.2% hexane, 2.5 L) to afford the desired urea as a white solid (230 g, 51%): mp 149-152 C.; 1H-NMR (DMSO-d6) delta 1.18 (s, 9H), 3.57 (s, 3H), 6.02 (s, 1H), 6.85 (d, J=6.0 Hz, 2H), 7.08 (d, J=9.0 Hz, 2H), 7.52 (d, J=9.0 Hz, 2H), 8.40 (d, J=6.0 Hz, 2H), 8.46 (s, 1H), 8.97 (s, 1H); FAB-LSIMS m/z 366 ((M+H)+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping