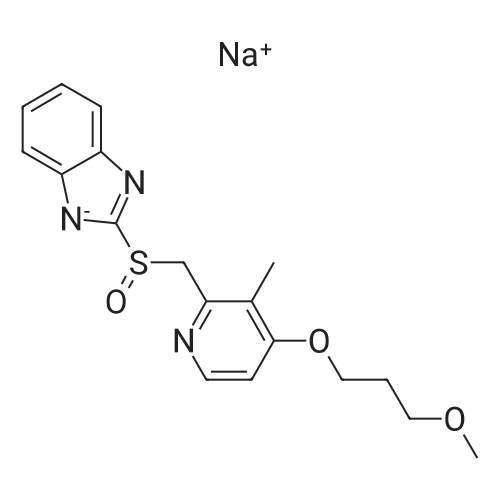
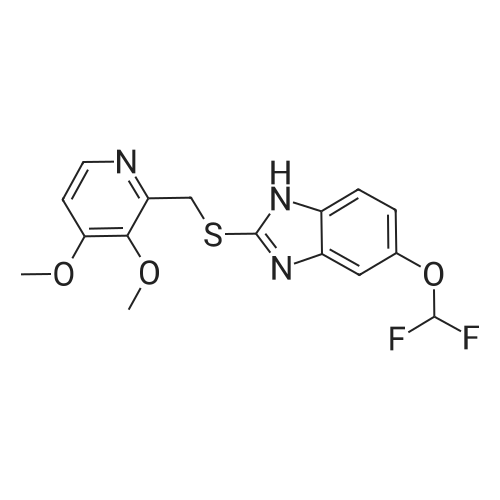
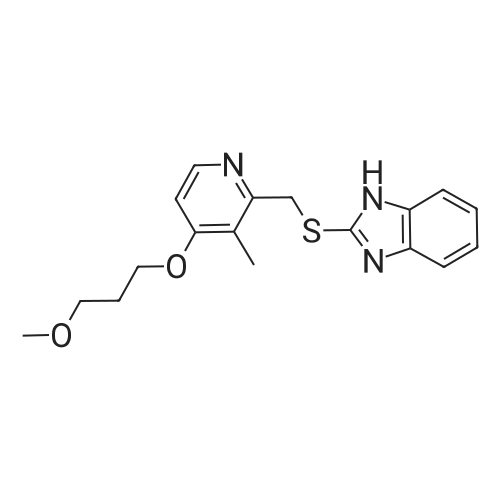
| 97.0 - 97.5% |
In Diethyl carbonate;Purification / work up; |
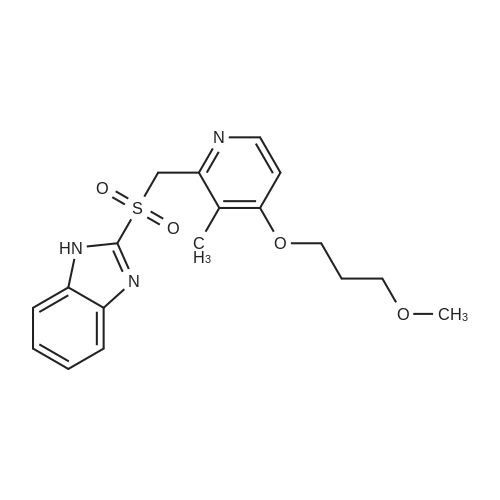
(Reference Example 5-8) The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized with diethyl carbonate (90 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.85 g) (HPLC purity 99.2%, yield 97.0%). The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized with diethyl carbonate (45 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (2.93 g) (HPLC purity 99.3%, yield 97.5%). |
| 97.0 - 97.5% |
In Diethyl carbonate;Crystallization;Purification / work up; |
Reference Example 5-8; The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized using diethyl carbonate (90 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyrid in-2-yl}methylthio]-1H-benzimidazole (4.85 g) (HPLC purity 99.2%, yield 97.0%).; Reference Example 6-8 The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized using diethyl carbonate (45 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (2.93 g) (HPLC purity 99.3%, yield 97.5%). |
| 96.0 - 97.0% |
In acetone;Purification / work up; |
(Reference Example 5-7) The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized with acetone (20 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.80 g) (HPLC purity 99.2%, yield 96.0%). The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized with acetone (9 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (2.91 g) (HPLC purity 99.3%, yield 97.0%). |
| 96.0 - 97.0% |
In ethyl acetate;Crystallization;Purification / work up; |
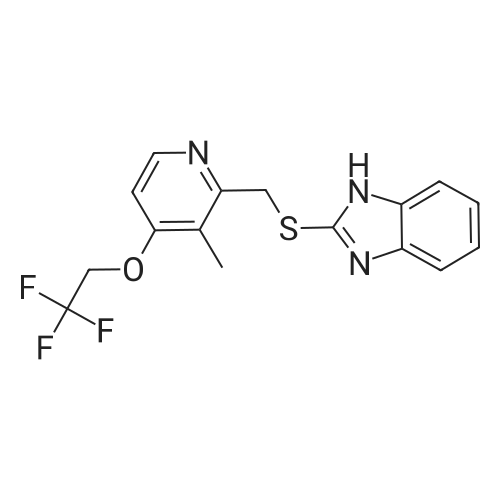
Reference Example 5-1; 2-[{4-(3-Methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole-producing Step 2-Chloromethyl-4-(3-methoxypropoxy)-3-methylpyridine (53.2 g (200 mmol)), denatured ethanol (320 ml), 2-benzimidazolethiol (30.2 g (201 mmol)), and sodium hydroxide (26.8 g (670 mmol)) were added together, and reaction was carried out for approximately 2 hours at 50 C. After it had been confirmed by TLC that the starting material had disappeared, vacuum concentration was carried out, and ethyl acetate (430 ml) and water (340 ml) were then added. After stirring and then leaving to stand, the aqueous layer was separated off. The organic layer was washed with a 10% sodium hydroxide aqueous solution (110 ml), and twice with water (110 ml), and then vacuum concentration was carried out, thus obtaining crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (69.0 g) (HPLC purity 98.7%, yield 101%). The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (5.00 g) was crystallized using ethyl acetate (25 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.80 g) (HPLC purity 99.2%, yield 96.0%).; Reference Example 6-2 The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized using ethyl acetate (12 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (2.91 g) (HPLC purity 99.3%, yield 97.0%). |
| 96.0 - 97.0% |
In acetone;Crystallization;Purification / work up; |
Reference Example 5-7; The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized using acetone (20 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.80 g) (HPLC purity 99.2%, yield 96.0%).; Reference Example 6-7 The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized using acetone (9 ml), and then filtration was carried out, thus obtaining 2-[4-(3-methoxypropoxy)-3-methylpyridin-2-yl }methylthio]-1H-benzimidazole (2.91 g) (HPLC purity 99.3%, yield 97.0%). |
| 92.8 - 93.5% |
In acetonitrile;Purification / work up; |
(Reference Example 5-5) The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized with acetonitrile (40 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.64 g) (HPLC purity 99.1 %, yield 92.8%). The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized with acetonitrile (21 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (2.81 g) (HPLC purity 99.1 %, yield 93.5%). |
| 92.8 - 93.5% |
In acetonitrile;Crystallization;Purification / work up; |
Reference Example 5-5; The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized using acetonitrile (40 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.64 g) (HPLC purity 99.1%, yield 92.8%).; Reference Example 6-5 The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized using acetonitrile (21 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (2.81 g) (HPLC purity 99.1%, yield 93.5%). |
| 91.0 - 92.5% |
In isopropyl alcohol;Purification / work up; |
(Reference Example 5-6) The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized with isopropyl alcohol (20 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.55 g) (HPLC purity 99.1%, yield 91.0%). The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized with diisopropyl alcohol (9 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (2.78 g) (HPLC purity 99.4%, yield 92.5%). |
| 91.2 - 94.5% |
In toluene;Purification / work up; |
The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized with toluene (15 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (2.84 g) (HPLC purity 99.1 %, yield 94.5%).(Reference Example 5-4) The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized with toluene (30 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.56 g) (HPLC purity 99.1%, yield 91.2%). |
| 91.0% |
In isopropyl alcohol;Crystallization;Purification / work up; |
Reference Example 5-6; The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized using isopropyl alcohol (20 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.55 g) (HPLC purity 99.1%, yield 91.0%). |
| 91.2 - 94.5% |
In toluene;Crystallization;Purification / work up; |
Reference Example 5-4; The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized using toluene (30 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.56 g) (HPLC purity 99.1%, yield 91.2%).; Reference Example 6-4 The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized using toluene (15 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (2.84 g) (HPLC purity 99.1%, yield 94.5%). |
| 90.0 - 93.0% |
In tert-butyl methyl ether;Purification / work up; |
(Reference Example 5-2) The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized with tert-butyl(methyl) ether (30 m), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (4.50 g) (HPLC purity 99.2%), yield 90.0%). The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized with tert-butyl(methyl) ether (12 ml), and filtered to obtain 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1 H-benzimidazole (2.79 g) (HPLC purity 99.2%, yield 93.0%). |
| 90.0 - 93.0% |
In tert-butyl methyl ether;Crystallization;Purification / work up; |
Reference Example 5-2; The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (5.00 g) obtained in Reference Example 5-1 was crystallized using tert-butyl(methyl) ether (30 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (4.50 g) (HPLC purity 99.2%, yield 90.0%).; Reference Example 6-3; The crude 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (3.00 g) obtained in Reference Example 6-1 was crystallized using tert-butyl(methyl) ether (12 ml), and then filtration was carried out, thus obtaining 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylthio]-1H-benzimidazole (2.79 g) (HPLC purity 99.2%, yield 93.0%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping