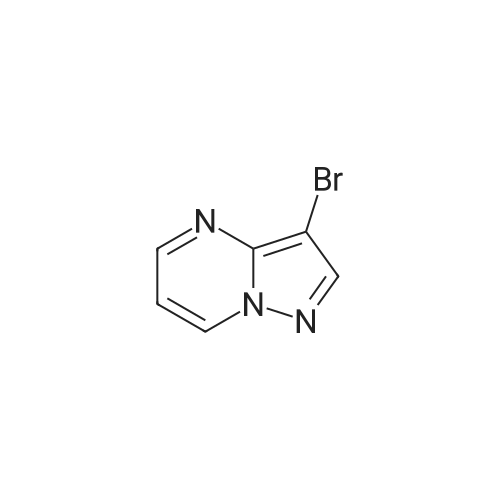
| 51.4% |
With potassium acetate;bis-triphenylphosphine-palladium(II) chloride; In 1,4-dioxane; at 20 - 100℃; |
To a stirred solution of compound 13f (27.8 mmol) in 1,4-dioxane (120 mL) was added B2Pm2 (126 mmol) and KOAc (101 mmol) at rt. The resulting mixture was purged with Ar for 45min, PdCl2(PPli3)2 (1.5 mmol) was added and the mixture again purged with Ar for 30min. The resulting mixture was heated to 1000C for 15h. The reaction <n="71"/>mixture was concentrated to obtain a viscous mass, which was charged over afluorosil plug, washed with pentane, followed by 60% EtOAc/Pet ether. The relevant fractions were concentrated to obtain a crude compound 13g as a pale yellow solid. The crude compound 13g was stirred with pentane (25mL) at -400C for 30min, filtered, washed with cold pentane (5mL) and dried under vacuum to obtain sufficiently pure compound (3.5g, 51.4%). TLC system: Ethyl acetate: Petroleum ether (2:3) Rf value: 0.4. (M + H): 246.3. |
|
With potassium acetate;tetrakis(triphenylphosphine) palladium(0); In 1,4-dioxane; at 100℃; for 20h;Inert atmosphere; Sealed vessel; |
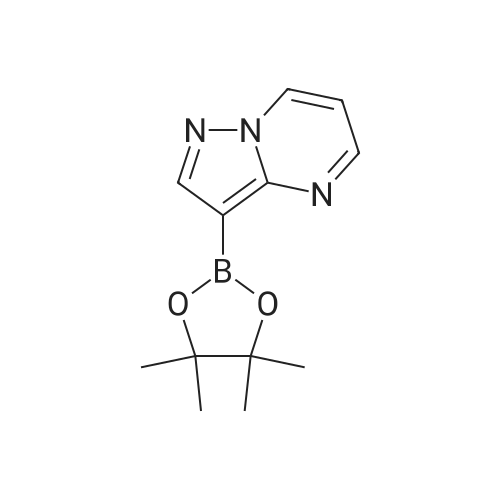
PREPARATION 107 3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazolo[1,5-a]pyrimidine [Show Image] A mixture of <strong>[55405-67-9]3-bromopyrazolo[1,5-a]pyrimidine</strong> (1.00 g, 5.1 mmol), potassium acetate (1.78 g, 18.1 mmol) and bis(pinacolato)diboron (5.77 g, 22.7 mmol) in 1,4-dioxane (20 mL) contained in a Schlenck vessel was submitted to three vacuum-argon cycles and bis(triphenylphosphine)palladium(II) dichloride (0.180 g, 0.26 mmol) was then added. The mixture was further submitted to three vacuum-argon cycles, sealed and then was stirred and heated to 100 C. After 20 hours, the reaction mixture was cooled, evaporated and then taken up in pentane and filtered through diatomaceous earth (Celite) and the filter cake was washed with a mixture of ethyl acetate/ether (3:2). The combined filtrate and washings were evaporated and the residue was stirred with n-pentane (15 mL) at -40 C for 30 minutes. The solid was filtered, washed with cold pentane and dried in vacuo to give the title compound (1.66 g, >100%) as a solid which was used without further purification. LRMS (m/z): 246 (M+1)+.1H NMR (300 MHz, CDCl3) delta ppm 1.26 (s, 12H), 6.89 (dd, 1H), 8.44 (s, 1H), 8.63 - 8.83 (m, 2H). |
|
With potassium acetate;tetrakis(triphenylphosphine) palladium(0); In 1,4-dioxane; at 100℃; for 20h;Inert atmosphere; Sealed vessel; |
PREPARATION 107 3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazolo[1,5-a]pyrimidine A mixture of <strong>[55405-67-9]3-bromopyrazolo[1,5-a]pyrimidine</strong> (1.00 g, 5.1 mmol), potassium acetate (1.78 g, 18.1 mmol) and bis(pinacolato)diboron (5.77 g, 22.7 mmol) in 1,4-dioxane (20 mL) contained in a Schlenck vessel was submitted to three vacuum-argon cycles and bis(triphenylphosphine)palladium(II) dichloride (0.180 g, 0.26 mmol) was then added. The mixture was further submitted to three vacuum-argon cycles, sealed and then was stirred and heated to 100 C. After 20 hours, the reaction mixture was cooled, evaporated and then taken up in pentane and filtered through diatomaceous earth (Celite) and the filter cake was washed with a mixture of ethyl acetate/ether (3:2). The combined filtrate and washings were evaporated and the residue was stirred with n-pentane (15 mL) at -40 C for 30 minutes. The solid was filtered, washed with cold pentane and dried in vacuo to give the title compound (1.66 g, >100%) as a solid which was used without further purification. LRMS (m/z): 246 (M+1)+.1H NMR (300 MHz, CDCl3) delta ppm 1.26 (s, 12H), 6.89 (dd, 1H), 8.44 (s, 1H), 8.63 - 8.83 (m, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping