| 65% |
With potassium hydrogencarbonate; In acetonitrile; at 20℃; |
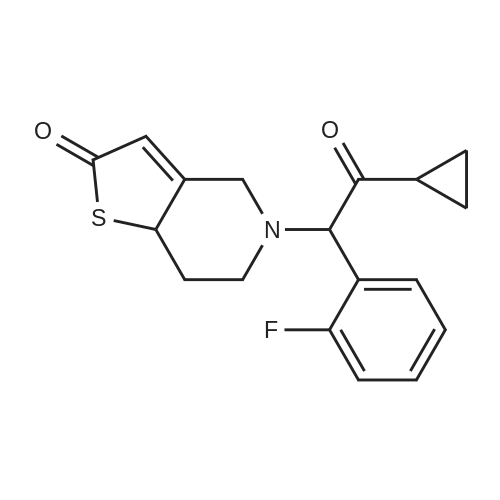
General procedure: To a stirred solution of methyl 2-bromo-2-(2-chlorophenyl)acetate (2a, 26.3 g, 0.1 mol) in CH3CN (500 mL) were added 5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one hydrochloride (1, 20.9 g, 0.11 mol) and potassium bicarbonate (30.0 g, 0.3 mol). The reaction was stirred at room temperature overnight.The reaction mixture was filtered and the liquid was concentrated under reduced pressure. |
| 35% |
In N-methyl-acetamide; potassium hydrogencarbonate; |
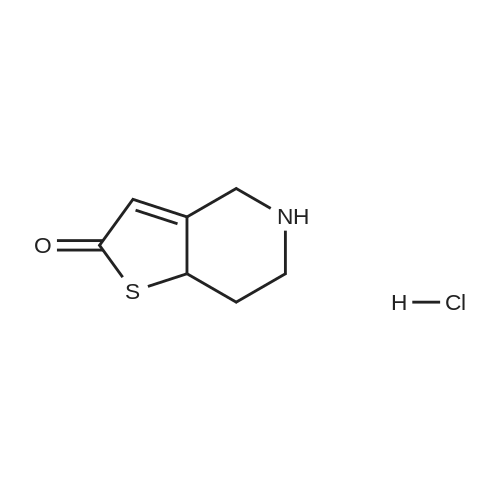
To a solution of alpha-cyclopropylcarbonyl-2-fluorobenzyl bromide (6.0 g) obtained above in dimethylformamide (20 ml) was added 2-oxo-2,4,5,6,7,7a-hexahydrothieno[3,2-c]pyridine hydrochloride (4.8 g), which was prepared according to the method described in EP 192535 (Japanese Patent Application Publication No. Sho 61-246186) and potassium bicarbonate (7.0 g). After stirring the mixture at room temperature for 2 hours the reaction mixture was partitioned between ethyl acetate and water. The ethyl acetate layer was washed with saturated aqueous sodium chloride solution, then dried over anhydrous magnesium sulfate, and evaporated under reduced pressure. After purification of the residue by chromatography on a silica gel column using toluene/ethyl acetate=3/1 as the eluant, the product was crystallized from diisopropyl ether to afford the desired product (2.6 g, yield 35%) as pale brown crystals. mp: 123-125 C.; 1H NMR (CDCl3) deltappm: 0.75-0.96 (2H, m), 0.99-1.14 (2H, m), 1.83-2.01 (1H, m), 2.02-2.17 (1H, m), 2.25-2.45 and 2.47-2.62 (total 2H, each m), 2.85 and 3.10 (total 2H, each d, J=12.0 Hz), 3.88-4.01 and 4.03-4.16 (total 2H, each m), 4.85 and 4.89 (total 1H, each s), 6.03 and 6.06 (total 1H, each s), 7.10-7.45 (4H, m); Mass (CI, m/z):332 (M++1), 262; Anal Calcd. for C18H18FNO2S: C, 65.23; H, 5.48; N, 4.23 Found: C, 65.09; H, 5.55; N, 4.20. |
| 35% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 2h; |
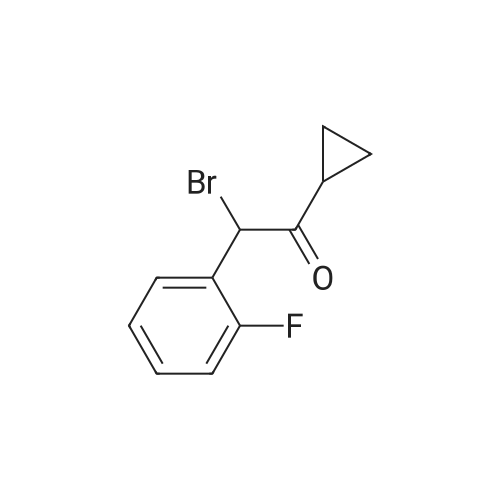
To a solution of cyclopropyl 2-fluorobenzyl ketone (8.7 g) obtained in part (a) in carbon tetrachloride (80 ml) was added N-bromosuccinimide (9.6 g) and benzoyl peroxide (0.5 g), then the mixture was heated under reflux for 6 hours. After the reaction, toluene was added to the reaction mixture and the resulting solid was filtered off. The filtrate was concentrated under reduced pressure. The residue was purified by chromatography on a silica gel column using toluene as the eluant to afford alpha-cyclopropylcarbonyl-2-fluorobenzyl bromide (8.5 g) as a yellow oil. To a solution of alpha-cyclopropylcarbonyl-2-fluorobenzyl bromide (6.0 g) obtained above in dimethylformamide (20 mL) was added 2-oxo-2,4,5,6,7,7a-hexahydrothieno[3,2-c]pyridine hydrochloride (4.8 g), which was prepared according to the method described in EP 192535 (Japanese Patent Application Publication No. Sho 61-246186) and potassium bicarbonate (7.0 g). After stirring the mixture at room temperature for 2 hours the reaction mixture was partitioned between ethyl acetate and water. The ethyl acetate layer washed with saturated aqueous sodium chloride solution, then dried over anhydrous magnesium sulfate, and evaporated under reduced pressure. After purification of the residue by chromatography on a silica gel column using toluene/ethyl acetate=3/1 as the eluant, the product was crystallized from diisopropyl ether to afford the desired product (2.6 g, yield 35%) as pale brown crystals. 1H NMR (CDCl3) delta ppm: 0.75-0.96 (2H, m), 0.99-1.14 (2H, m), 1.83-2.01 (1H, m), 2.02-2.17 (1H, m), 2.25-2.45 and 2.47-2.62 (total 2H, each m), 2.85 and 3.10 (total 2H, each d, J=12.0 Hz), 3.88-4.01 and 4.03-4.16 (total 2H, each m), 4.85 and 4.89 (total 1H, each s), 6.03 and 6.06 (total 1H, each s), 7.10-7.45 (4H, m). Mass (Cl, m/z):332 (M ++1), 262; Anal Calcd. for C18H18FNO2S: C, 65.23; H, 5.48; N, 4.23. Found: C, 65.09; H, 5.55; N, 4.20. |
|
With sodium carbonate; In N,N-dimethyl-formamide; at 0 - 5℃; for 3h;Product distribution / selectivity; |
Example-19: Preparation of 5-(alpha-cyclopropylcarbonyI-2-fluorobenzyI)-2-oxo-2,4,5,6,7,7a-hexahydrothieno[3, 2-c] pyridine.; The title compound is prepared analogues manner to example- 18 using the 5,6,7,7a-tetrahydro-4H-thieno[3,2-c]-pyridin-2-one hydrochloride in place of 5,6,7, 7a-tetrahydro-4H-thieno[3,2-c]-pyridin-2 -one p-touenesulfonate. Yield: 1.25 grams |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 5 - 25℃; for 2.66667h; |
EXAMPLE 6: PREPARATION OF 5-[2-CYCLOPROPYL-1 -(2- FLUOROPHENYL)-2-OXOETHYL]5,677a-TETRAHYDRO-THIENO[3,2- c]PYRIDIN-2(4H)-ONE. alpha-Cyclopropyl carbonyl 2-fluorobenzyl bromide (50.0 g) and dimethyl formamaide (50 mL) are charged into a round bottom flask. Potassium carbonate (50 g) is charged with stirring to the above suspension. The reaction mixture is cooled to 5 0C. A solution of 5,6,7,7a-tetrahydro-thieno[3,2-c]pyridine-2-(4H)-one hydrochloride (45.0 g) in dimethylformamide (50 mL) is added over 25 minutes at 5 0C and stirred for 30 minutes. The reaction mixture is further stirred for 1 hour, 45 minutes at 25 0C. The reaction mixture is decomposed by adding chilled water (500 mL) and then water (300 mL) is decanted from the reaction mixture. Ethyl acetate (500 mL) is charged to the obtained reaction mixture. The layers are separated. The obtained ethyl acetate layer is washed with 5% saturated aqueous sodium chloride solution (2*100 mL), and then dried over sodium sulfate. The organic solvent is concentrated completely under reduced pressure at 52 0C. The reaction crude is extracted into ethyl acetate (300 mL) and the obtained ethyl acetate layer is concentrated completely at 55 0C to afford 20.2 g of the title compound. |
|
|
Example-19: Preparation of 5-(a-cyclopropylcarbonyI-2-fluorobenzyl)-2-oxo- 2,4,S,6,7,7a-hexahydro thieno[3,2-c] pyridine compound of formuIa-7:A mixture of 5,6,7,7a-tetrahydro-4H-thieno[3,2-c]-pyridin-2-one-hydrochloride (100 grams), potassium carbonate (63.5 grams) and acetonitrile (1000 ml) was stirred for 30 minutes at 25-30C. 2-bromo-l-cyclopropyl-2-(2-fluorophenyl) ethanone (66 grams) in acetonitrile (30 ml) was added to the reaction mixture and stirred for 7 hours at 25-30C. The reaction mixture was filtered and removed the precipitated solid. The filtrate was distilled off completely under reduced pressure; ethyl acetate followed by cyclohexane was added to it. The reaction mixture was stirred for 25 minutes at 40-45C. The reaction mixture was cooled to 25-30C and stirred for an hour. The reaction mixture was filtered and solvent from the filtrate was distilled off completely under reduced pressure to get the title compound.Yield: 70 grams |
|
With potassium carbonate; In acetonitrile; at 25 - 30℃; for 7.5h; |
Example 19Preparation of 5-(alpha-cyclopropylcarbonyl-2-fluorobenzyl)-2-oxo-2,4,5,6,7,7a-hexahydro thieno[3,2-c] pyridine Compound of Formula-7 A mixture of 5,6,7,7a-tetrahydro-4H-thieno[3,2-c]-pyridin-2-one-hydrochloride (100 grams), potassium carbonate (63.5 grams) and acetonitrile (1000 ml) was stirred for 30 minutes at 25-30 C. 2-bromo-1-cyclopropyl-2-(2-fluorophenyl) ethanone (66 grams) in acetonitrile (30 ml) was added to the reaction mixture and stirred for 7 hours at 25-30 C. The reaction mixture was filtered and removed the precipitated solid. The filtrate was distilled off completely under reduced pressure; ethyl acetate followed by cyclohexane was added to it. The reaction mixture was stirred for 25 minutes at 40-45 C. The reaction mixture was cooled to 25-30 C. and stirred for an hour. The reaction mixture was filtered and solvent from the filtrate was distilled off completely under reduced pressure to get the title compound. |
|
|
84.01 g of 5,6,7,7a-Tetrahydro-4H-thieno[3,2-c]pyridin-2-one hydrochloride and 0.84 1 of acetonitrile were mixed in a 2 liter 4 neck round bottom flask. The mixture was cooled down to -5-0 C. In addition, 102.5 g of 2-Fluoro-alpha-cyclopropylcarbonyl benzyl bromide were added to the reactor and it was further stirred for 10 minutes at - 5-0 C. Next, 84.8 ml of a methanolic ammonia solution (16% w/v) were added dropwise to the mixture over a period of 2 hours and it was further stirred for 1 hour. Subsequently, 24.4 ml more of methanolic ammonia solution were added dropwise over a period of 1 hour. Afterwards, the mixture was quenched in 1.556 1 of water and 20.55 ml of concentrated HCl. Next, the pH of the mixture was adjusted to 7.0 with 102.7ml of a 10% (w/v) solution of sodium bicarbonate. The reaction mass was extracted with 1.027 1 of ethyl acetate. The aqueous layer was extracted with 513 ml of ethyl acetate. The layers were separated. Both ethyl acetate layers were mixed, washed with 345ml of brine solution and dried with 75 g of sodium sulphate. Lastly, the ethyl acetate layer was distilled out under vacuum at 50-55 C to give 125g of the title compound as a brown coloured semi solid. Yield: 95.00%). Purity (HPLC): 88.6% |
| 7.9 g |
With potassium carbonate; In water; acetonitrile; at 20℃; for 2.25h; |
Cyclopropylcarbonyl-2-fluorobenzyl bromide and 7.2 g of 2-oxo-2,4,5,6,7,7a-hexahydrothieno[3,2-c]pyridine hydrochloride 5.4 g of water is added to 0.72 mL of water. 3.23 g of potassium carbonate was added thereto, followed by stirring at room temperature for 1 hour. 3.23 g of potassium carbonate was added thereto, and the mixture was stirred at room temperature for 1 hour, then 3.23 g of potassium carbonate was added again and stirred. The reaction solution was sampled according to the reaction time (15 min, 30 min, 1 h, 1 h 15 min, 1 h 30 min, 2 h, 2 h 15 min), and then the ethyl acetate and water were added to separate the organic layer. The compound thus obtained was confirmed by HPLC analysis. The conversion ratios of 2-oxoprazole, according to the reaction time, are shown in Table 4 below. After confirming that the reaction was completed, the by-product was filtered and washed with ethyl acetate. 57.7 mL of ethyl acetate and 57.7 mL of water were added to the filtrate, and the organic layer was separated after stirring. The organic mixed solution was washed with an aqueous ammonium chloride solution, water and an aqueous sodium chloride solution, and then concentrated under reduced pressure to obtain 7.9 g of 2-oxoprazole glycolated oil. |
|
With sodium carbonate; at 40℃; for 10h;Large scale; |
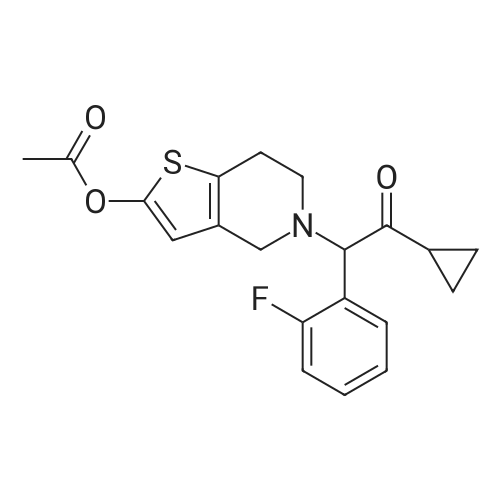
To 52 1 methyl-isobutyl-ketone 17.8 kg anhydrous sodium-carbonate and 10.0 kg 96% 5,6,7,7a-tetrahydrothieno[3,2-c]pyridine-2(4H)-one hydrochloride of Formula (TV) are measured then 13.4 kg 96% 2-bromo-l-cyclopropyl-2-(2-fluorophenyl)ethanone of Formula (III) is added. The mixture is stirred for 10 h at 40C then cooled, and the inorganic compounds are filtered off. To the filtrate 300 g dimethylamino-pyridine and 22.4 1 triethyl- amine are added, cooled to 0-5C then 11.0 1 acetic anhydride is added dropwise. After 2 h, to the mixture 40 1 ethyl acetate then 40 1 water are added dropwise. After separation the organic phase is dried then evaporated to dryness. 2x40 1 ethanol is poured onto the reminder then evaporated. The oily remainder is solved in 40 1 ethanol. The crystalline material is stirred first at 20-25C for 1 h then at 0-5C for further 1 h then filtered, washed with 8 1 cool ethanol and dried. (0082) Thus 11.4 kg crude prasugrel base is obtained. (Yield: 61 %.) |
|
|
At 0 C,Add 38.3 ga and 350 ml of dichloromethane to a 1 L three-neck round bottom flask, and add 77.6 g of DIPEA at a temperature control of 0 C. After the completion of the dropwise addition, stir for 0.5 h, continue to add 51.4 gb, and stir the reaction for 3 h after the end of the addition. 51.7 g of DIPEA was added dropwise, and then 20.4 g of acetic anhydride was added dropwise to the reaction system to control the temperature of the reaction system to 0 C. After the completion of the dropwise addition, the reaction was further stirred for 4 hours, the reaction was completed, and 350 ml of water was added for extraction, and the organic phase was dried over anhydrous sodium sulfate. After drying, filtration and concentration under reduced pressure gave 62.1 g of a yellow solid, which was determined to be d, yield was 83.2%, and HPLC purity was 99.3%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping