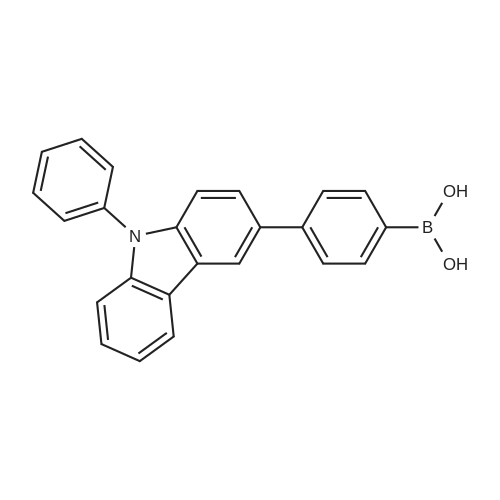
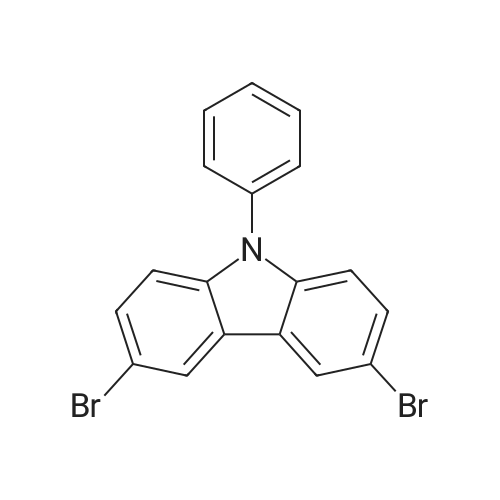
| 90% |
With potassium carbonate;palladium diacetate; tris-(o-tolyl)phosphine; In ethanol; water; toluene; at 85℃; for 9h;Inert atmosphere; |
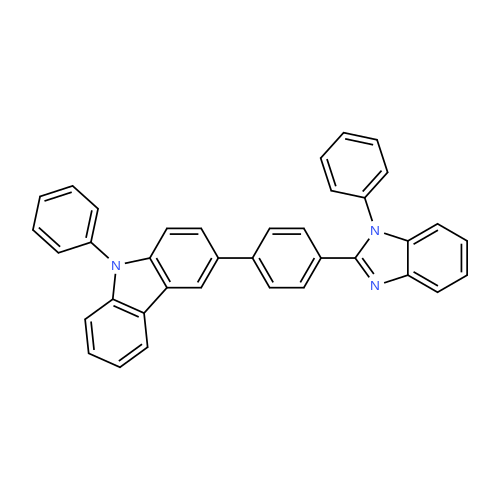
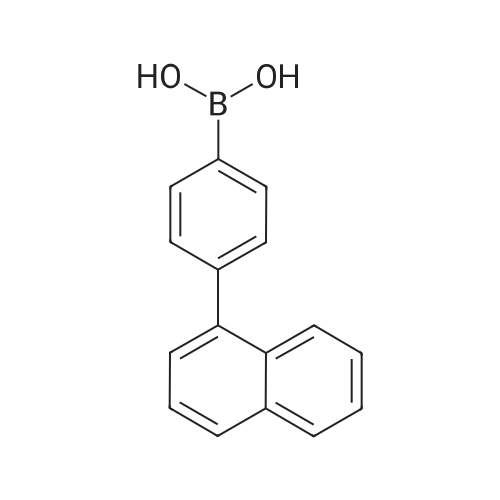
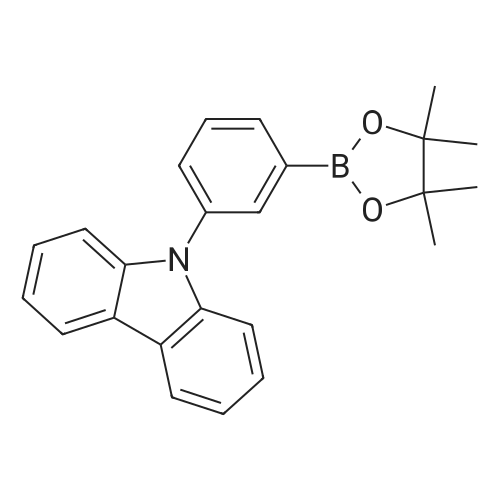
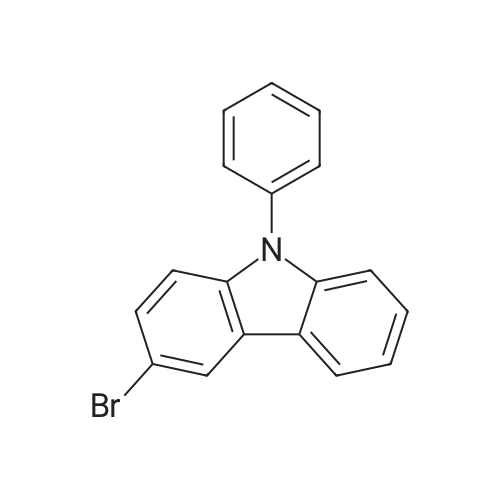
Synthesis Example 1; In a 200-mL three-neck flask, a mixture of 5.0 g (15.5 mmol) of 3-bromo-9-phenyl-9H-carbazole, 4.2 g (17.1 mmol) of 4-(1-naphthyl)-phenylboronic acid, 38.4 mg (0.2 mmol) of palladium(II) acetate, 104 mg (0.3 mmol) of tris(2-methylphenyl)phosphine, 50 mL of toluene, 5 mL of ethanol, and 30 mL of a potassium carbonate aqueous solution (2 mol/L) was deaerated while being stirred under reduced pressure, and then heated and stirred in a nitrogen atmosphere at 85 C. for 9 hours to be reacted.After the reaction, 500 mL of toluene was added to the reaction mixture solution, and an organic layer of the mixture solution was filtrated through Florisil (Catalog No. 540-00135, produced by Wako Pure Chemical Industries, Ltd.), alumina (neutral, produced by Merck Ltd), and Celite (Catalog No. 531-16855, produced by Wako Pure Chemical Industries, Ltd.). The obtained filtrate was washed with water, and magnesium sulfate was added thereto so that moisture was adsorbed. This suspension was filtrated to obtain a filtrate. The obtained filtrate was concentrated and purified by silica gel column chromatography. At this time, a mixed solvent of toluene and hexane (toluene:hexane=1:4) was used as a developing solvent for the chromatography. The obtained fraction was concentrated, and methanol was added thereto. The mixture was irradiated with ultrasonic waves and then recrystallized to give 6.24 g of white powder that was an objective substance in a yield of 90%. The reaction scheme of Synthesis Example 1 above is shown in (F1-1). The Rf values of the objective substance and 3-bromo-9-phenyl-9H-carbazole were respectively 0.42 and 0.58, which were obtained by silica gel thin layer chromatography (TLC) (with a developing solvent of ethyl acetate and hexane in a 1:10 ratio).The compound obtained in Synthesis Example 1 was examined by a nuclear magnetic resonance (NMR) method. The measurement data are shown below.1H NMR (CDCl3, 300 MHz): delta (ppm)=7.30-7.35 (m, 1H), 7.44-7.67 (m, 14H), 7.76 (dd, J=8.7 Hz, 1.8 Hz, 1H), 7.84-7.95 (m, 4H), 8.04 (d, J=7.8, 1H), 8.23 (d, J=7.8, 1H), 8.46 (d, J=1.5, 1H).FIGS. 7A and 7B are 1H NMR charts. Note that FIG. 7B is a chart showing an enlarged part of FIG. 7A in the range of 7.0 ppm to 9.0 ppm. The measurement results confirmed that 3-[4-(1-naphthyl)-phenyl]-9-phenyl-9H-carbazole (abbreviation: PCPN) that was the objective substance was able to be obtained. |
| 90% |
With palladium diacetate; potassium carbonate; tris-(o-tolyl)phosphine; In ethanol; water; toluene; at 85℃; for 9h;Inert atmosphere; |
In a 200 mL three-neck flask, a mixture of 5.0 g (15.5 mmol) of 3-bromo-9-phenyl-9H-carbazole, 4.2 g (17.1 mmol) of 4-(1-naphthyl)-phenylboronic acid, 38.4 mg (0.2 mmol) of palladium(II) acetate, 104 mg (0.3 mmol) of tri(ortho-tolyl)phosphine, 50 mL of toluene, 5 mL of ethanol, and 30 mL of a 2 mol/L aqueous potassium carbonate solution was degassed while being stirred under reduced pressure, and reacted by being stirred and heated at 85 C. for 9 hours under a nitrogen atmosphere. (0523) After the reaction, 500 mL of toluene was added to this reaction mixture solution, and the organic layer of this mixture solution was filtered through Florisil (produced by Wako Pure Chemical Industries, Ltd., Catalog No. 540-00135), alumina, and Celite (produced by Wako Pure Chemical Industries, Ltd., Catalog No. 531-16855). The obtained filtrate was washed with water, and magnesium sulfate was added thereto so that moisture was adsorbed. This suspension was filtered to obtain a filtrate. The obtained filtrate was concentrated and purified by silica gel column chromatography. At this time, a mixed solvent of toluene and hexane (toluene:hexane=1:4) was used as a developing solvent for the chromatography. The obtained fraction was concentrated, and methanol was added thereto. The mixture was irradiated with ultrasonic waves and then recrystallized to give 6.24 g of a white powder in a yield of 90%, which was the object of the synthesis. (0524) This compound was identified as 3-[4-(1-naphthyl)-phenyl]-9-phenyl-9H-carbazole (abbreviation: PCPN), which was the object of the synthesis, by nuclear magnetic resonance (1H-NMR) spectroscopy. (0525) 1H NMR data of the obtained substance are as follows: 1H NMR (CDCl3, 300 MHz): delta (ppm)=7.30-7.35 (m, 1H), 7.44-7.67 (m, 14H), 7.76 (dd, J=8.7 Hz, 1.8 Hz, 1H), 7.84-7.95 (m, 4H), 8.04 (d, J=7.8, 1H), 8.23 (d, J=7.8, 1H), 8.46 (d, J=1.5, 1H). |
| 90% |
With palladium diacetate; potassium carbonate; tris-(o-tolyl)phosphine; In ethanol; water; toluene; at 85℃; for 9h;Inert atmosphere; |
In a 200 mL three-necked flask3-bromo-9-phenyl-9H-carbazole(11.5 mmol) of 4- (1-naphthalene) phenylboronic acid, 38.4 mg (0.2 mmol) of palladium (II) acetate, 104 mg (0.3 mmol) of tris (o-tolyl) , 50 mL of toluene, 5 mL of ethanol and 2 ml of a 20 mol / L aqueous solution of potassium carbonate were degassed with stirring under reduced pressure, and then stirred at 85 C for 9 hours under nitrogen atmosphere to cause reaction.After the reaction, 500 mL of toluene was added to the reaction mixture, and the organic layer of the mixed solution was treated with magnesium silicate (Japan Wako Pure Chemical Industries, Ltd., catalog number: 540-00135), alumina and diatomaceous earth (Manufactured by Wako Pure Chemical Industries, Ltd., catalog number: 531-16855). The resulting filtrate was washed with water and magnesium sulfate was added to adsorb moisture. The suspension was filtered to obtain a filtrate. The resulting filtrate was concentrated and then purified by silica gel column chromatography. In this case, as a developing solvent for silica gel column chromatography, a mixed solvent of toluene and hexane (toluene: hexane = 1: 4) was used. The resulting rinsing was concentrated, and methanol was added and ultrasonic waves were applied, followed by recrystallization, and a white powder of the desired product was obtained at a yield of 6.24 g and a yield of 90%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping