| 82 - 84% |
With N-Bromosuccinimide; Azo-bis(2,4-dimethyl-valeronitril); In dichloromethane; at 45 - 50℃; for 2 - 5h;Industry scale; |
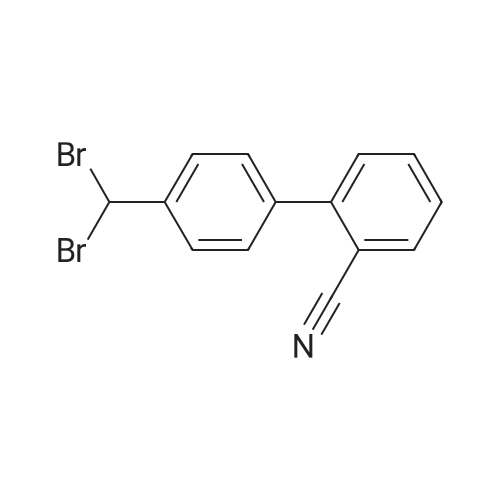
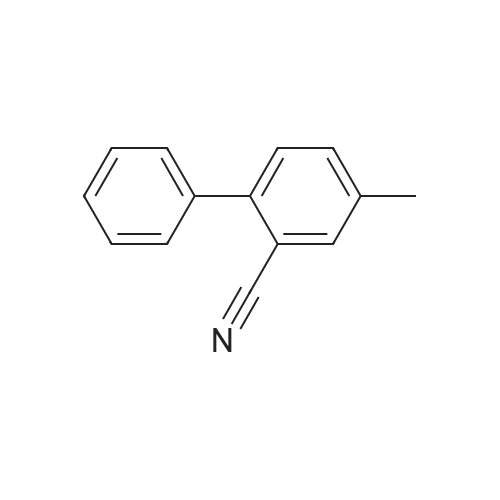
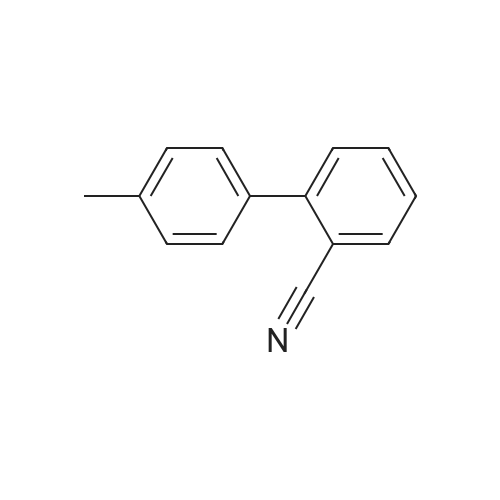
23 g of 2-(4-methylphenyl)benzonitrile [MPB], 22g of N-bromosuccinic acid imide [NBS] and 47 mg of 2,2'-azobis(2,4-dimethylvaleronitrile) were suspended in 44 ml of dichoromethane, and the reaction was allowed to proceed at 45 to 50C for 5 hours under stirring. 46 ml of water was added, and the layers were separated to obtain organic layer (The same procedure was carried out three times totally).. The organic layer was concentrated, and 50 ml of acetonitrile was added.. The solution was concentrated again, and 50 ml of acetonitrile was added thereto to obtain 116 g of a solution of 2-(4-bromomethylphenyl)benzonitrile [BMB] in acetonitrile (yield calculated from a quantitated value of 2-(4-bromomethylphenyl)benzonitrile: 84 %). 30.1 g of methyl 2-tert-butoxycarbonylamino-3-nitrobenzoate [BAN], 40.8 g of potassium carbonate and 160 ml of acetonitrile were added to the acetonitrile solution in which unreacted 2-(4-methylphenyl)benzonitrile [MPB] and 2-(4,4-dibromomethylphenyl)benzonitrile as an analogue of BMB were contaminated, and the reaction was allowed to proceed at about 82 C for about 5 hours under stirring.. After cooling to room temperature, the precipitated crystals were filtered off, and the filtrate was concentrated to give methyl 2-[N-t-butoxycarbonyl-N-[(2'-cyanobiphenyl-4-yl)methyl]amino]-3-nitrobenzoate [BBN].. The concentrated material was dissolved in 190 g of methanol, and 106 g of concentrated hydrochloric acid was added dropwise thereto.. The resulting solution was heated to a reflux temperature over 2 hours, and stirred for 2 hours under reflux, whereby, the reaction was allowed to proceed.. The reaction solution was cooled, and precipitated crystals were collected by filteration and dried to give 35.1 g of methyl 2-[N-(2'-cyanobiphenyl-4-yl)methylamino]-3-nitrobenzoate [MBN] (yield relative to 2-(4-methylphenyl)benzonitrile [MPB] was 76.1 %). Reference Example 4 (1)Synthesis of 4-(2-bromomethylphenyl)benzonitrile [BMB] 2-(4-Methylphenyl)benzonitrile [MPB] (271 kg), N-bromosuccinic acid imide [NBS] (256 kg), 2,2'-azobis(2,4-dimethylvaleronitrile) [ABN-V] (543 kg) and methylene chloride (680 kg) were stirred at 45 to 50 C under reflux, and the reaction was allowed to proceed until an area percentage of 4-(2-boromomethylphenyl)benzonitrile [BMB] with HPLC became 82 % or more (about 2 to 5 hours).. After the reaction solution was cooled to 38 to 42 C, methylene chloride (250 kg) was added.. Water (540 L) was added, the layers were separated, the resulting aqueous layer was extracted with 50 kg of methylene chloride, and the organic layers were combined (this procedure was repeated three times totally).. The methylene chloride layer was concentrated to about 700 L (about 2.5-fold volume of MPB) under atmospheric pressure (inner temperature: about 46 C).. acetonitrile (about 640 kg) was added thereto, the resulting solution was concentrated to about 1100 L under reduced pressure (about 200 to 450 MmHg) while maintaining the inner temperature at 45 to 55 C (desirably 45 to 50 C).. Then, acetonitrile (about 480 kg) was added, and the resulting solution was concentrated to about 500 L under reduced pressure (about 200 to 450 MmHg) while maintaining the inner temperature at 45 to 55 C (desirably 45 to 50 C).. acetonitrile (about 480 kg) was added to the residue, whereby the amount of solution came to about 1100 L, to give a solution containing 2-(4-bromomethylphenyl)benzonitrile [BMB], unreacted 2-(4-methylphenyl)benzonitrile [MPB] and 2-(4,4-dibromomethylphenyl)benzonitrile as an analogue of BMB in acetonitrile. |
| 10 - 20%; 80 - 90% |
With sodium bromate; hydrogen bromide; In dichloromethane; water; at 0 - 20℃;UV-irradiation; |
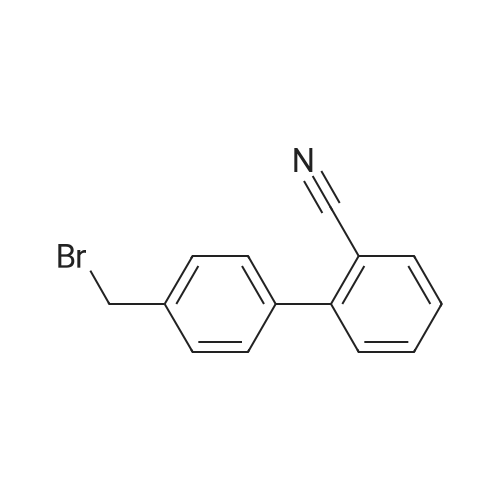
A jacketed 1,000 mL 3-neck flask was charged with 4'-methylbiphenyl-2-carbonitrile (Compound 1, 100.0 g) and CH2Cl2 (500 mL) under nitrogen. To a 500 mL Erlenmeyer flask with magnetic stirrer, sodium bromate (NaBrO3; 31.2 g) was dissolved in water (170 mL). The NaBrO3 solution was transferred to the 1,000 mL flask and the reaction mixture was cooled to about 5 C. or less. Aqueous HBr solution (48%, 105.0 g) was added to the 1,000 mL flask and the resulting reaction mixture was recycled though a UV lamp reactor. The reaction mixture was kept at 0-20 C. and the recycling was continued until the reaction was deemed complete by HPLC. Optionally, additional sodium bromate and hydrogen bromide may be added. The relative amounts of Compound 2 and Compound 3 were about 80-90% and about 10-20% respectively. Aqueous sodium metabisulfite solution (2.0 g of in 10 mL water) was added to the reaction mixture. Allow the phases to settle and the methylene chloride phase was washed with water and used in the next step without further purification. |
| 69% |
With sodium bromate; azobis(2-cyanobutane); bromine; In water; chlorobenzene; at 70 - 80℃; for 6h; |
Production Example; Chlorobenzene (652.5 g) was added with 4'-methyl-2-cyanobiphenyl (416.2 g, 2.251 mole) and sodium bromate (57.41 g, 0.38 mole) dissolved in water (109 g), and the mixture was warmed at 75 to 80C. A solution of 2,2'-azobis(2-methylbutyronitrile) (8.31 g, 0.043 mole) dissolved in monochlorobenzene (87 g) was dropped together with bromine (217.5 g, 1.116 mole) into the mixed solution above at 70 to 80C over 5 hours, then leaving it for 1 hour with maintaining its temperature. The solution was set still at about 80C and then subjected to phase separation. The organic layer was added with 217.5 g of water, washed at about 80C and then subjected to phase separation. The separated solution was concentrated under a reduced pressure to eliminate 91.4 g of chlorobenzene. The residual solution was added with 304.5 g of chlorobenzene to filter at 75 to 80C by a filter pre-coated with 11 g of diatomaceous earth. Washing with 208.8 g of chlorobenzene, then the filtrate was cooled down after confirming no precipitation of crystal observed therein. The filtrate was added with 450 mg of seed crystal at the temperature thereof of about 50C, and stirred at 55C for 6 hours. Then, this solution was cooled down in the rate of 10C/hour, followed by stirring at the temperature thereof of 30 to 35C for 5 hours and at the temperature thereof of 0 to -5C for 10 hours, and then being subjected to filtration. By rinsing the crystal with heptane, 404.6 g of 4'-bromomethyl-2-cyanobiphenyl was obtained in the yield of 69 %. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping