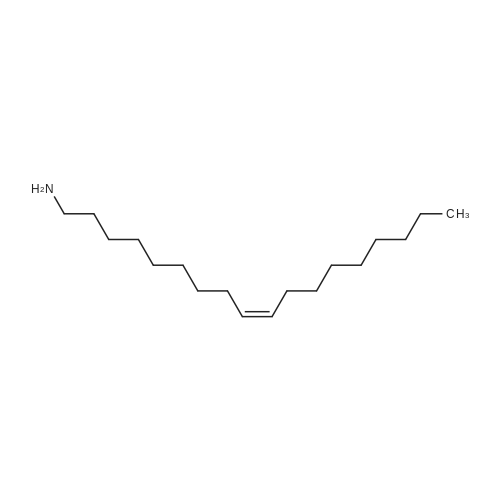
| 3.2 g |
|
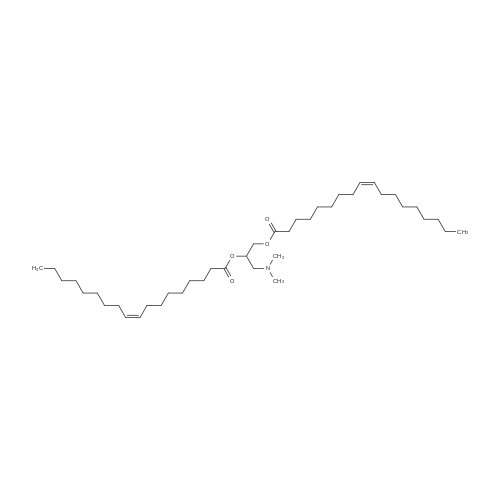
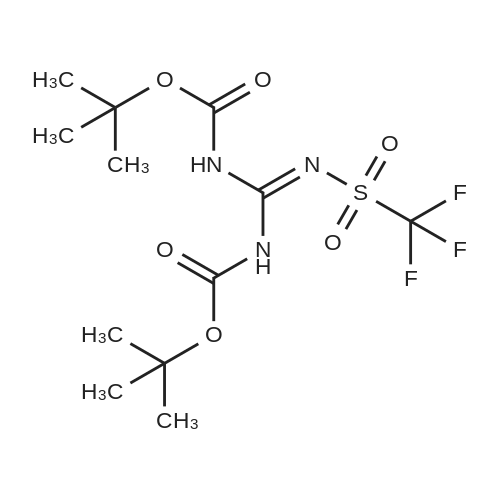
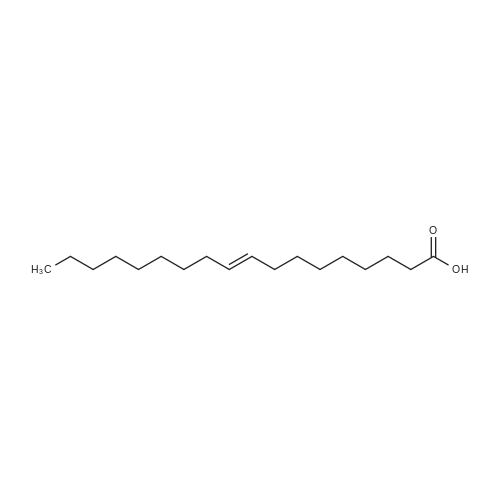
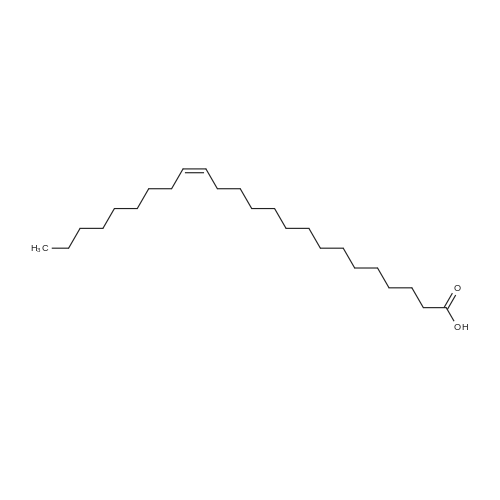
Example 20 Preparation of C18(oleic)-norArg-C18 N-(4-guanidino-1-oxo-1-(octadecylamino)butan-2-yl)octadec-9-enamide (0607) Fmoc-Dab(Boc)-resin. To 5 g of 2-chlorotrityl chloride resin with 1.3 mmol/g substitution (Novabiochem, 01-64-0114) in 50 ml of dry DCM in 60 ml reaction vessel for solid phase synthesis, 5.726 g (13 mmol, 2 eq) of Fmoc-Dab(Boc)-OH (Mw=440.5, (Fmoc-(N-gamma-Boc)-L-alpha,gamma-diaminobutyric acid, AnaSpec, 28246) and 2.26 ml (13 mmol, 2.0 eq) of DIPEA (Aldrich, Mw=129.2, d=0.74) were added. (0608) Dab(Boc)-resin. After 3 hrs the resin was washed 3× with DCM/MeOH/DIPEA (17:2:1), 3×DCM, 2×DMF, 3×DCM and Fmoc group was deprotected with 50 ml of 20percent piperidine/DMF twice for 15 min. (0609) C18:1-Dab(Boc)-resin. After Fmoc deprotection the resin was washed with 3×DCM, 2×MeOH and 3×DCM and for the coupling reaction 3.7 g (13 mmol) of oleic acid (Sigma, Mw=282.47, d=0.891), 5.37 g (13 mmol) of HCTU (Mw=413.7) and 2.62 ml (13 mmol) of DIPEA (Mw=129.2, d=0.74) in 50 ml of DMF were added. (0610) After 1 hr of reaction the resin was washed with 3×DCM, 2×MeOH and 3×DCM and progress of reaction was checked by Kaiser test which was negative (no free amine groups present). (0611) C18:1-Dab(Boc)-OH (Mw=566.56) was cleaved from the resin by 1percent TFA/DCM (5×50 ml for 2 min was filtered to flask with 2 ml 10percent pyridine/MeOH) and solvent was evaporated. (0612) C18:1-Dab(Boc)-C18:1 (Mw=734.95). Second coupling was carried out in solution. To the oily residue from the previous reaction, 3.725 g (9.75 mmol) of oleyl amine (Sigma, Mw=267.49, 70percent), 4.033 g (9.75 mmol) of HCTU (Mw=413.7) and 1.69 ml (9.75 mmol) of DIPEA (Mw=129.2, d=0.74) in 50 ml of DMF were added. After 4 hr 100 ml of AcOEt was added and organic layer was washed in separatory funnel with 3×0.5 M HCl, 3×10percent NaCO3 and 3×NaCl. AcOEt layer was dried with anhydrous MgSO4 and evaporated. (0613) C18:1-Dab-C18 (Mw=635.9). To the oily residue from the previous reaction, 100 ml of 80percent TFA/DCM 2.5percent TIS was added and after 20 min solvent was evaporated. (0614) C18:1-norArg(diBoc)-C18:1 (Mw=874.13). The residue was dissolved in 50 ml of DCM and pH was adjusted to 9 with TEA. 2.348 g (6 mM, 1 eq) of 1,3-Di-Boc-2-(trifluoromethylsulfonyl)guanidine (Mw=391.36, Aldrich 15033) was added and after 4 hrs DCM was evaporated. (0615) C18:1-norArg-C18:1 (Mw=674.1) To the oily residue from the previous reaction 100 ml of 95percent TFA/DCM 2.5percent TIS was added and after 3 hrs solvent was evaporated. Crude product was purified on TELEDYNE Isco CombiFlash Rf instrument using 48 g normal phase silica gel column, 100percent DCM for 3 CV (column volume) and 0-20percent MeOH for 10 CV, detection 214 nm, flow 45 ml/min. DCM/MeOH was evaporated and residue was precipitated by 0.1M HCl. Yield: 3.2 g. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping