|
With oxalyl dichloride;N,N-dimethyl-formamide; In dichloromethane; at 0℃; |
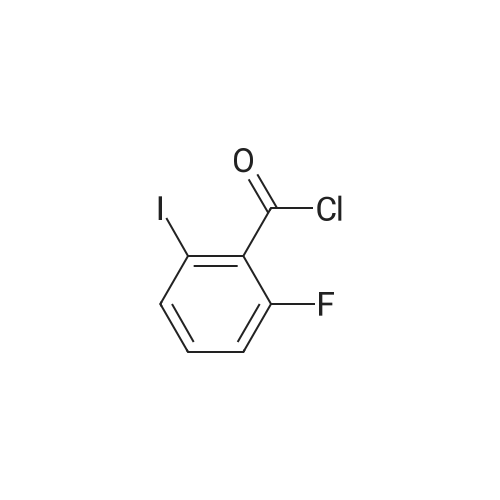
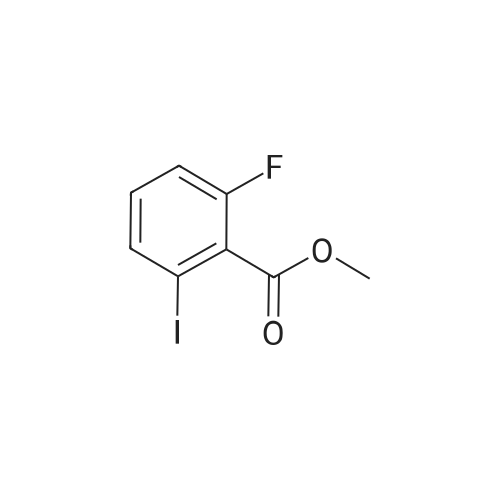
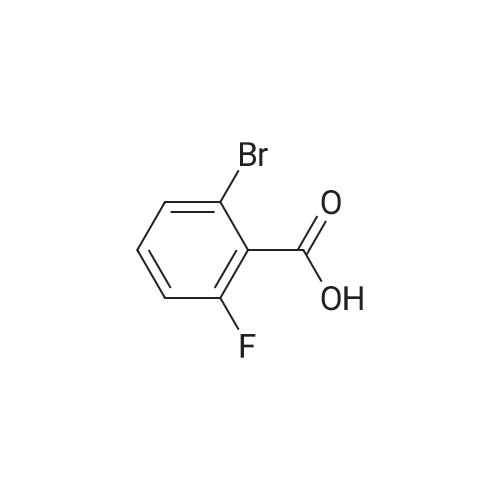
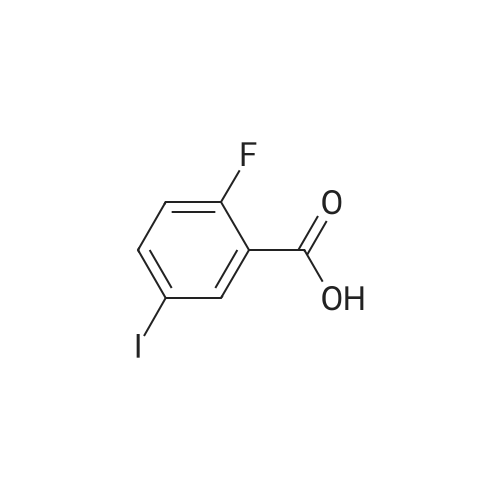
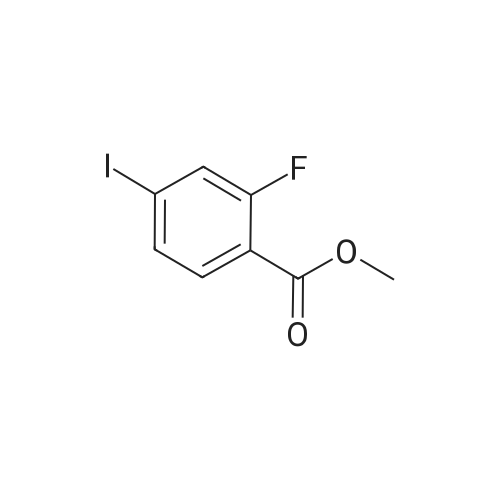
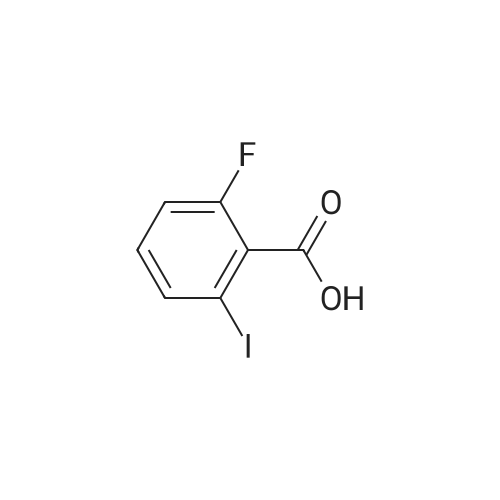
To a heterogeneous mixture of <strong>[111771-08-5]2-fluoro-6-iodobenzoic acid</strong> (1 .51 g, 5.66 mmol) at 0 C in DCM (28 mL) was added oxalyl chloride (0.635 mL, 7.36 mmol) followed by DMF (0.15 mL). Gas evolution commenced immediately and after 5 min the ice bath was removed. When gas evolution had ceased and the mixture was homogeneous an aliquot was removed and quenched with MeOH. Formation of the methyl ester was confirmed by HPLC and the mixture was concentrated in vacuo. The viscous liquid was dissolved in fresh DCM (28 mL) and treated with solid N-hydroxyacetamidine (503 mg, 6.79 mmol) in several portions followed by TEA (1 .2 mL, 8.49 mmol) at 0 C. After stirring for 14 h at ambient temperature the mixture was washed with saturated aqueous NaHCO3 solution. The combined organic extracts were dried over Na2SO4, filtered andconcentrated in vacuo. Chromatography (Hex to 100% EtOAc/Hex) afforded the desired product as a colorless oil (1 .57 g, 86%). MS (ESI) mass calculated for C9H8FIN2O2, 321 .96; m/z found, 323.0. 1 H NMR (500 MHz, CDCI3): 7.70 - 7.65 (m, 1 H), 7.15 - 7.1 1 (m, 2H), 4.87 (br s, 2H), 2.06 (s, 3H). |
|
With oxalyl dichloride; In dichloromethane; N,N-dimethyl-formamide; at 0 - 20℃; |
Step A: (Z)-N'-((2-Fluoro-6-iodobenzoyl)oxy)acetimidamide. To a heterogeneous mixture of <strong>[111771-08-5]2-fluoro-6-iodobenzoic acid</strong> (1 .51 g, 5.66 mmol) at 0 C in DCM (28 mL) was added oxalyl chloride (0.635 mL, 7.36 mmol) followed by DMF (0.15 mL). Gas evolution commenced immediately and after 5 min the ice bath was removed. When gas evolution had ceased and the mixture was homogeneous an aliquot was removed and quenched with MeOH. Formation of the methyl ester was confirmed by HPLC and the mixture was concentrated in vacuo. The viscous liquid was dissolved in fresh DCM (28 mL) and treated with solid N-hydroxyacetamidine (503 mg, 6.79 mmol) in several portions followed by TEA (1 .2 mL, 8.49 mmol) at 0 C. After stirring for 14 h at ambient temperature the mixture was washed with saturated aqueous NaHCO3 solution. The combined organic extracts were dried over Na2SO4, filtered andconcentrated in vacuo. Chromatography (Hex to 100% EtOAc/Hex) afforded the desired product as a colorless oil (1 .57 g, 86%). MS (ESI) mass calculated for C9H8FIN2O2, 321 .96; m/z found, 323.0. 1 H NMR (500 MHz, CDCI3): 7.70 - 7.65 (m, 1 H), 7.15 - 7.1 1 (m, 2H), 4.87 (br s, 2H), 2.06 (s, 3H). |
|
With oxalyl dichloride; In dichloromethane; at 20℃; for 1h;Inert atmosphere; |
Oxalyl chloride (2.5 ml, 28.66 mmol, 1.5 eq.) was added at room temperature to a suspension of <strong>[111771-08-5]2-fluoro-6-iodobenzoic acid</strong> (5.1 g, 19.17 mmol, 1 eq.) in 40 ml of dry DCM under nitrogen atmosphere. A few drops of dry DMF were added and the mixture was stirred at room temperature for 1 h. The solvent was removed by distillation. The obtainedresidue was taken up in acetonitrile, which was distilled again, to remove excess acidity. The resulting brown oil was dissolved in dry DCM and the resulting solution was drop- wise added to a suspension of potassium carbonate (7g, 50.6mmol, 2.6 eq.) in 20 ml of dry methanol. The reaction mixture was then stirred at room temperature overnight.The next morning the solids were filtered away and washed with DCM. The volume of theliquid phase was reduced by vacuum distillation. The resulting slurry was taken up inDCM and filtered again.The clear liquid phase was then dried over sodium sulphate, filtered and evaporated dodryness to give 4.27 g (15.25 mmol) of (D77) as a yellow oil.MS (ESI) mlz: 219 [M+H].1H NMR (ODd3) 5 ppm = 7.78 -7.60 (m, 1 H), 7.28 (s, 1 H), 7.14 (dd, J = 3.7, 8.1 Hz, 2 H), 4.00 (s, 3 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping