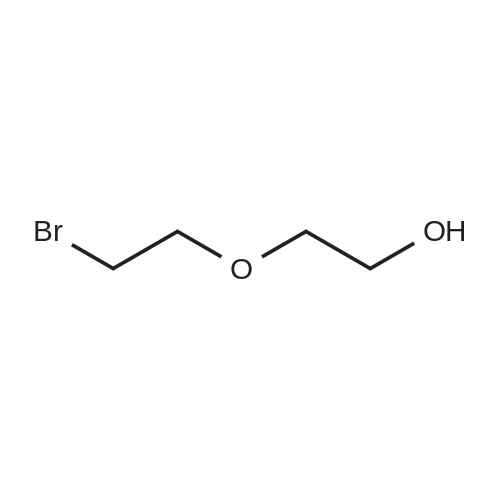
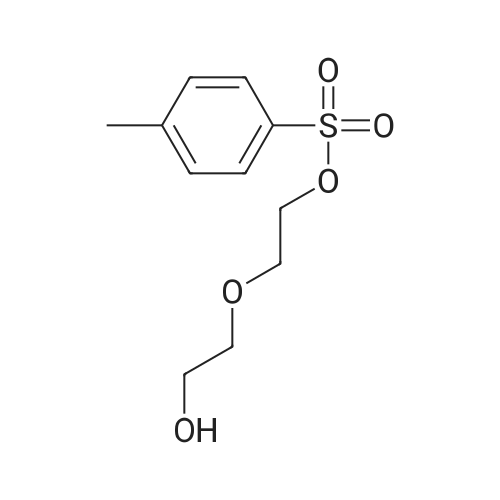
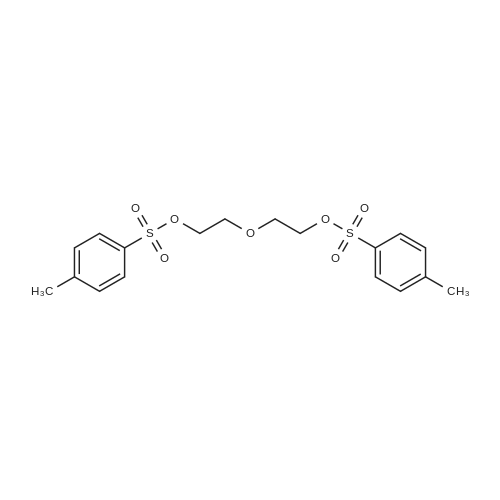
| 90.6% |
With sodium hydroxide; In tetrahydrofuran; water; at 20℃;Cooling; |
Add 95.07 g of diethylene glycol in THF to a 500 mL three-necked flask.The mixture was stirred and cooled under an ice salt bath to obtain a viscous liquid. Add NaOH solution (NaOH 5.46g;Water 30 mL) was stirred. Under ice water bath, a solution of TsCl in THF (TsCl 16.58 g, THF 70 mL) was added dropwise. After the addition was completed, stirring was continued for 2 hours under ice salt bath.Then stir at room temperature. After the reaction is completed, the stirring is stopped.The reaction solution was poured into 200 mL of ice water and extracted with CH 2 Cl 2 (100 mL×2).The organic phase was washed with water (50 mL×2), dried over anhydrous magnesium sulfate and filtered.Rotating to obtain a transparent viscous liquid, which is Compound A, the yield is 90.6%; |
| 89% |
With sodium hydroxide; In tetrahydrofuran; water; for 6h; |
In a 250 mL three-necked flask, 92.40 g of diethylene glycol and 5.22 g of 30 mL aqueous NaOH solution were added.THF 100ml, TsCl 16.6g, after 6h reaction,The mixture was poured into ice water, extracted with CH2C12, the organic phase was washed with water, dried over anhydrous magnesium sulfate,Filtration of the compound F 20.29 g was obtained by filtration, yield 89%. |
| 87% |
|
General procedure: Oligo ethylene glycol (2 eq, 40 mmol) was dissolved intetrahydrofuran (20 mL) at 0 oC. Sodium hydroxide (1.5 eq,30 mmol, 1.2 g) in 15 mL H2O was added drop wise to thesolution of oligo ethylene glycol. After stirring for 30 min, ptoluenesulfonylchloride (1 eq, 20 mmol, 3.8 g) was dissolvedin tetrahydrofuran (15 mL) at 0 oC. Oligoethyleneglycol was added drop wise in the solution of ptoluenesulfonylchloride. After the addition was completed,the aqueous solution was treated with HCl 10% and extractedwith dichloromethane. The organic layer was washedwith distilled water and dried over MgSO4. After the solventwas removed, the residue was purified by column chromatography(ethyl acetate/n-hexane = 1:1 v/v) Diethylene glycol monotosylate (4a): This compoundwas obtained in 87% yield as colourless oil; ir: 3417 (OH),1353 and 1176 (S=O) cm-1; 1H-NMR (CDCl3): 7.72 (d, J =8.25 Hz, 3H); 7.29 (d, J = 8 Hz, 2H); 4.12 (t, J = 4.5 Hz,2H); 3.63-3.57 (m, 3H); 3.45 (t, J = 4.75 Hz, 2H); 3.18-3.14(m, 2H); 2.38 (s, 3H). 13C-NMR (CDCl3,): 144.9; 132.8;129.8; 128.2; 72.4; 69.3; 68.4; 61.4; 21.5.Anal. calc. forC11H16O5S1: C, 50.75; H, 6.18, S, 12.31. Found: C, 50.73; H,6.21; S, 12.36. |
| 65% |
With potassium hydroxide; In tetrahydrofuran; at 20℃; for 5h;Cooling with ice; |
Compound 1-1 (166 g, 1.57 mol) was dissolved in 500 mL of tetrahydrofuran, potassium hydroxide (32 g, 571 mmol) was added in an ice bath, and then p-toluenesulfonyl chloride 1-2 (100 g, 0.52 mol) was added in batches, and the mixture was stirred at room temperature for 5 hours. The reaction solution was poured into 1200 mL of water, (400 mL) and extracted twice with ethyl acetate. The extract liquid was combined and washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure and purified by silica gel column chromatography, with an eluent (petroleum ether:ethyl acetate=1:1), to obtain the target product 1-3 (55 g, 65%) as a colorless oil, LC-MS: m/z=261 [M+H]+. |
| 60% |
With triethylamine; In dichloromethane; at 20℃; |
Diethylene glycol (7.0 g, 66.0 mmol) was dissolved in 10 mL of dichloromethane solution, Et3N (13.3 g, 132.2 mmol) was added, and a solution of TsCl (15.1 g, 79.1 mmol) in dichloromethane was added dropwise under ice-cooling. After the completion of the dropwise addition, the mixture was transferred to room temperature and stirred overnight. After adjusting the pH of the solution to 7 with 6 mol/L HCl, 10 mL of water was added, and the mixture was extracted three times with 30 mL of dichloromethane, and the organic phases were combined and dried over anhydrous sodium sulfate. Column chromatography (EA) gave 10 g of a yellow liquid product, yield 60%. |
| 60% |
With triethylamine; In dichloromethane; at 20℃;Cooling with ice; |
Diethylene glycol (7.0 g, 66.0 mmol) was dissolved in 10 mL of dichloromethane solution, Et3N (13.3 g, 132.2 mmol) was added, and TsCl (15.1 g, 79.1 mmol) in dichloromethane was added dropwise on ice. .After the completion of the dropwise addition, the mixture was transferred to room temperature and stirred overnight.After adjusting the pH of the solution to 7 with 6 mol/L HCl, 10 mL of water was added, and the mixture was extracted three times with 30 mL of dichloromethane, and the organic phases were combined and dried over anhydrous sodium sulfate.Column chromatography (EA) gave 10 g of a yellow liquid product, yield 60%. |
| 56% |
With potassium iodide; silver(l) oxide; In dichloromethane; at 20℃; for 12h; |
j00460j A solution of 2,2?-oxybis(ethan-i-ol) 1 (5 g, 47.1 mmol) in DCM (50 mL) wascharged with silver oxide (10.3 g, 70.7 mmol), potassium iodide (1.6 g, 0.09 mmol), and tosyl chloride (9 g, 47.1 mmol) and stirred at room temperature for 12 h. The reaction mixture was filtered through a pad of celite and the filtrate was concentrated in vacuo resulting in a crude compound which was purified by chromatography on silica gel, eluting with 5% methanol in DCM to give 6.9 g, 56% yield, of the title compound as a colorless thick oil. ?H NMR (400 MHz, CDC13): oe = 7.81 (d, J= 8.38 Hz, 2H), 7.35 (d, J= 7.94 Hz, 2H), 4.20 (t, J= 4.63 Hz, 2H), 3.65 -3.73 (m, 4H), 3.54 (d, J= 4.41 Hz, 2H), 2.45 (s, 3H), 1.94 (br. s, 1H). |
| 51% |
|
To a solution of 2-(2-hydroxyethoxy)ethanol (55.66 g, 524.53 mmol, 49.70 mL, 2 eq) in tetrahydrofuran (500 mL) was added sodium hydride (6.29 g, 157.27 mmol, 60% purity, 0.6 eq) at 0 C and stirred for 0.5 hour under nitrogen. Then the mixture was added p- toluenesulfonyl chloride (50 g, 262.26 mmol, 1 eq), warmed to 25 C and stirred for 6 hours. The mixture was poured into saturated ammonium chloride solution (200 mL) and stirred for 15 minutes. The aqueous phase was extracted with ethyl acetate (200 mL x 2). The combined organic phase was washed with brine (200 mL), dried with anhydrous anhydrous sodium sulfate, filtered and concentrated in vacuum. The residue was purified by silica gel chromatography (Petroleum ether/Ethyl acetate=l0/l to 1/1) to afford 2-(2-hydroxyethoxy)ethyl 4- methylbenzenesulfonate (35 g, 134.46 mmol, 51 % yield) as a yellow oil. LC/MS (ESI) m/z. 261.0 [M+l] +; 1H-NMR (400MHz, CDCb) d 7.76 - 7.72 (m, 2H), 7.28 (d, / = 8.0 Hz, 2H), 4.15 - 4.02 (m, 2H), 3.66 - 3.55 (m, 4H), 3.49 - 3.44 (m, 2H), 2.38 (s, 3H). |
| 47% |
|
(1042) Step A (1043) Diethyleneglycol (1.25 g, 11.8 mmol) was dissolved in water (3.5 mL) and sodium hydroxide (0.768 g, 19.2 mmol) was added. The mixture was stirred at room temperature until a clear solution was obtained. The mixture was cooled to 0 C. and a solution of 4-toluene sulfonylchloride (2.27 g, 11.9 mmol) in tetrahydrofuran (30 mL) was dropwise added over a period of 20 minutes. After the addition was completed, the reaction mixture was stirred at 0 C. for 1 hour. The reaction mixture was diluted with ethyl acetate (150 mL) and water (40 mL). The organic phase was separated, dried over Na2SO4, filtered and the solvents were evaporated under reduced pressure. The residue was purified on HP-Sil SNAP cartridges using a Biotage Isolera One purification system employing an dichloromethane/methanol gradient (100/0->100/0) to afford the more polar title compound as a colorless oil (1.47 g, 47%). The less polar bis-tosyl derivative was discarded. (1044) 1H-NMR (400 MHz, CDCl3) delta=7.80 (d, 2H), 7.35 (d, 2H), 4.22-4.18 (m, 2H), 3.72-3.66 (m, 4H), 3.55-3.51 (m, 2H), 2.45 (s, 3H). |
| 46% |
With triethylamine; In dichloromethane; at 25℃; for 12h; |
Diethylene glycol (3.18 g, 30 mmol) and triethylamine (TEA) (8.0 mL) were dissolved in dichloromethane (60 mL). Then, tosyl-chloride (5.70 g, 30 mmol) was added in one portion. The resulting mixture was stirred at 25 oC for 12 h. After washing with KHSO4 (1 M, 40 mL) and NaHCO3 (5%, 40 mL), respectively and drying over Na2SO4, the crude product was obtained by evaporation and subsequently purification by column chromatography over silica gel (dichloromethane) to obtain the target product 4 as a colorless oil (3.61 g, 13.8 mmol, 46%) |
| 40% |
With triethylamine; In dichloromethane; at 25℃; for 16h;Inert atmosphere; |
A solution of diethylene glycol (5.01 g, 47.21 mmol), / olucncsulfonyl chloride (4.50 mg, 23.6 mmol) and triethylamine (8.55 mL, 61.37 mmol) in DCM (200 mL) was stirred at 25 C for 16 h. The reaction was added to FLO (30 mL) and extracted with DCM (2 x 50 mL). The organic layer was separated, dried with over Na2S04 and concentrated under vacuum. The residue was purified by silica gel chromatography (petroleum ether :EtO Ac 10:1 to 1 : 1) to afford 2-(2-hydroxyethoxy)ethyl 4-methylbenzenesulfonate (2.95 g, 40%) as a light yellow oil; LC-MS 283.0 [M+Na]+. |
| 39% |
In dichloromethane; at 16℃; for 18h; |
To a stirred solution of diethylene glycol (compound 3A, 10.0 g, 94.2 mmol), Et3N (2.86 g, 28.3 mmol) in DCM (50 mL) was added a solution of 4-methylbenzenesulfonyl chloride (4.49 g, 23.6 mmol) in DCM (50 mL) slowly at 16 C. Then the reaction mixture was stirred for 18 hours at 16 C. TLC (PE:EA=1: 1) showed that the reaction was completed. The reaction mixture was washed with saturated NaHC03(50 mL x 2). The organic layer was dried over anhydrous Na2S04and concentrated in vacuo to give the crude product. The crude product was purified by silica gel chromatography (PE:EA=5: 1-1: 1) to give 2-(2-hydroxyethoxy)ethyl 4- methylbenzenesulfonate (compound 3B, 2.4 g, 39%) as colorless oil. MS: calc'd 278 (M+H20)+, measured 278 (M+H20)+. |
| 36% |
With triethylamine; In dichloromethane; at 0 - 20℃;Inert atmosphere; |
To a solution of commercially available diethyleneglycol 23 (4.0 g,37.7 mmol) and p-toluenesulfonyl chloride (7.55 g, 39.6 mmol) stirring in anhydrousCH2Cl2 under an Ar atmosphere at 0C was slowly added Et3N (15.8mL,113.1 mmol). After stirring overnight at rt, the reaction was diluted withCH2Cl2 and poured into a saturated aq. NaHCO3 solution. The aq. layer wasextracted three times with CH2Cl2, and the combined organic portions werewashed with brine, dried over MgSO4, and concentrated under reduced pressure.The product mixture was purified by silica gel column chromatography togive 24 (3.56 g, 36%) as colorless oil. 1H NMR (400 MHz, CDCl3): delta 7.83-7.76 (d,J = 8.31 Hz, 2H), 7.37-7.30 (d, J = 7.34 Hz, 2H), 4.21-4.15 (m, 2H), 3.70-3.63(m, 4H), 3.54-3.49 (m, 2H), 2.46-2.40 (s, 3H). 13C NMR (100 MHz, CDCl3): delta144.95, 129.84, 127.92, 72.46, 68.54, 61.61, 21.63. |
| 29.5% |
With triethylamine; In dichloromethane; for 0.5h;Cooling with ice; |
Diethylene glycol 69 (20 g, 0.188 mol) was dissolved in CH2Cl2 (500 mL) followed by the addition of TEA (29 mL, 0.207 mol), then cooled with an ice bath. TsCl (20 g, 0.094 mol) in CH2Cl2 (100 mL) was added drop-wise during a period of 30 min. Slowly warmed to room temperature and kept reacting for another 1 hour. The solvent was removed and purified by silica gel chromatography eluting with 20% EA/hexane, then 66% EA/hexane to give 14.4 g (29.5%) of diethylene glycol mono-Ts 70. Then, the obtained compound above diethylene glycol mono-Ts 70 (12.61 g, 44.2 mmol) and N-Boc-hydroxylamine (8.25 g, 62 mmol) were dissolved in CH2Cl2 (400 mL) followed by the addition of DBU (13.5 mL, 88.4 mol). After 48 hours reaction, the reaction was diluted with 400 mL of CH2Cl2, washed with 1N HCl (100 mL, twice), H2O (100 mL) and brine (200 mL), the organic layer was dried over anhydrous MgSO4 and evaporated. The crude was purified by silica gel chromatography eluting with 33% EA/hexane, then 50% EA/hexane containing 5% MeOH to give 1.5 g (16.1%) of diethylene glycol mono-Ts 71. The product was dissolved in CH2Cl2 (70 mL) followed by the addition of TEA (2.96 mL, 21.3 mmol), then cooled with an ice bath. TsCl (2 g, 9.4 mmol) in CH2Cl2 (20 mL) was added drop-wise during a period of 30 min. Slowly warmed to room temperature and kept reacting for another 1 hour. The solvent was removed and purified by silica gel chromatography eluting with 10% EA/hexane, then 40% EA/hexane to give 1.31 g (49%) of OTs-diethylene glycol hydroxylamine-Boc 72. Finally, OTs-diethylene glycol hydroxylamie-Boc 72 (1.3 g, 3.48 mmol) was dissolved in anhydrous ethanol (50 mL) followed by the addition of sodium azide (680 mg, 10.4 mmol), then refluxed overnight. The solvent was removed, diluted with EA (200 mL), washed with H2O (50 mL) and brine (50 mL), the organic layer was dried over anhydrous MgSO4 and evaporated. The crude was purified by silica gel chromatography eluting with 10% EA/hexane, then 40% EA/hexane to give 728 mg (85%) of Azido-diethylene glycol hydroxylamie-Boc 73. |
| 24% |
With potassium iodide; silver(l) oxide; In dichloromethane; at 0 - 25℃; for 1.66667h;Inert atmosphere; |
To a solution of diethyleneglycol (20.0 g, 17.9 ml, 188 mmol) in DCM (200 ml) under argon were added tosyl chloride (53.9 g, 283 mmol) and silver oxide (65.5 g, 283 mmol) and the suspension was cooled to 0-5 C. Then potassium iodide (6.26 g, 37.7 mmol) was added over 10 min in 5 portions. After the addition was completed, the reaction was warmed to 20- 25C and stirred for l.5h. The dark grey suspension was filtered, the cake washed with DCM (100 ml), and the filtrate concentrated under vacuum at 40 C. A yellowish non-transparent emulsion was obtained which was purified by flash chromatography (Si02, Hept/EtOAc 5% to 90%) to obtain the desired product (11.6 g,. 24%) as a colorless oil. LC-MS: m/z = 415.2 [M+H]+. |
| 22% |
With triethylamine; In dichloromethane; at 40℃; for 12h; |
General procedure: Ethylene glycol (500 mg, 8.1 mmol) and triethylamine (0.54 mL, 4.0 mmol) were dissolved in 7.5 mL dry CH2Cl2, and then TsCl (384 mg, 2.0 mmol) was added into the mixture. The reaction mixture was stirred 12 h at 40C. The resulting mixture was diluted with CH2Cl2, washed with saturated aqueous NH4Cl , NaHCO3 and NaCl successively, and dried over anhydrous Na2SO4. After concentrated in vacuo, the residue was purified by column chromatography (CH2Cl2/MeOH = 20:1) to give 15a (260mg, 23%) as colorless oil. |
|
With triethylamine; In dichloromethane; at 25℃; for 1h;Inert atmosphere; |
Synthesis of 2-(2-hydroxyethoxy)ethyl 4-methylbenzenesulfonate (Compound 3) Into a 2000-mL 3-necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen was placed a solution of 2-(2-hydroxyethoxy)ethan-l-ol (Compound 2, 42.4 g, 399.55 mmol, 1.00 equiv) in dichloromethane (1000 mL) and triethylamine (27.9 g, 275.72 mmol, 0.25 equiv). To the above was added p-toluenesulfonyl chloride (19.1 g, 100.18 mmol, 0.50 equiv). After stirred for 1 h at 25C, the resulting mixture was washed with 1x500 mL of aq. potassium hydrosulfate (1M) and 1x500 mL of aq. sodium bicarbonate (5%) respectively. The organic layer was dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column and eluted with dichloromethane/methanol (100: 1). This resulted in 2-(2-hydroxyethoxy)ethyl 4- methylbenzenesulfonate (Compound 3) as a colorless oil. |
|
With triethylamine; In dichloromethane; at 25℃; for 1h;Inert atmosphere; |
Synthesis of 2-(2-hydroxyethoxy)ethyl 4-methylbenzenesulfonate (Compound A3) Into a 2000-mL 3 -necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen was placed a solution of 2-(2-hydroxyethoxy)ethan-l-ol (A2, 42.4 g, 399.55 mmol, 1.00 equiv) in dichloromethane (1000 mL) and triethylamine (27.9 g, 275.72 mmol, 0.25 equiv). To the above was added p-toluenesulfonyl chloride (19.1 g, 100.18 mmol, 0.50 equiv). After stirred for 1 h at 25C, the resulting mixture was washed with 1x500 mL of aq. potassium hydrosulfate (1M) and 1x500 mL of aq. sodium bicarbonate (5%) respectively. The organic layer was dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column and eluted with dichloromethane/methanol (100: 1). This resulted in 2-(2-hydroxyethoxy)ethyl 4- methylbenzenesulfonate (Compound A3) as a colorless oil. |
|
With triethylamine; In dichloromethane; at 25℃; for 1h;Inert atmosphere; |
Into a 2000-mL 3 -necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen was placed a solution of 2-(2-hydroxyethoxy)ethan-l-ol (2, 42.4 g, 399.55 mmol, 1.00 equiv) in dichloromethane (1000 mL) and triethylamine (27.9 g, 275.72 mmol, 0.25 equiv). To the above was added p-toluenesulfonyl chloride (19.1 g, 100.18 mmol, 0.50 equiv). After stirred for 1 h at 25C, the resulting mixture was washed with 1x500 mL of aq. potassium hydrosulfate (1M) and 1x500 mL of aq. sodium bicarbonate (5%) respectively. The organic layer was dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column and eluted with dichloromethane/methanol (100: 1). This resulted in 2-(2- hydroxyethoxy)ethyl 4-methylbenzenesulfonate (3) as a colorless oil. |
|
With pyridine; In dichloromethane; at 0℃; |
To a solution of diethylene glycol (270 muL, 2.8 mmol) and pyridine (227.9 muL, 2.8 mmol) in CH2Cl2was added dropwise of a solution of tosyl chloride (805.7 mg, 2.8 mmol) in CH2Cl2at 0 C and stirred overnight. The solvent was removed under pressure to give pale yellow oil which was purified by siliga gel column chromatography with EtOAc as eluent to obtain diethylene glycol monotosylate as a colorless oil. Cassiarin A (60.5 mg, 0.3 mmol) and potassium carbonate (200.8 mg, 1.4 mmol) in DMF was stirred at room temperature for 1 h. The mixture was added diethylene glycol monotosylate (84.6 mg, 0.3 mmol), stirred and refluxed for 2 days and quenched by adding water, then extracted with EtOAc. The organic phase was washed with saturated NaCl, dried over Na2SO4, concentrated under pressure and purified by recrystallization from EtOAc to give5b.The product was characterized by spectroscopic methods. |
|
With triethylamine; In dichloromethane; at 25℃; for 1h;Inert atmosphere; |
Synthesis of 2-(2-hydroxyethoxy)ethyl 4-methylbenzenesulfonate (Compound A3)Into a 2000-mL 3 -necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen was placed a solution of 2-(2 -hydroxy ethoxy)ethan-l-ol (A2, 42.4 g, 399.55 mmol, 1.00 equiv) in dichloromethane (1000 mL) and triethylamine (27.9 g, 275.72 mmol, 0.25 equiv). To the above was added p-toluenesulfonyl chloride (19.1 g, 100.18 mmol, 0.50 equiv). After stirring for 1 h at 25C, the resulting mixture was washed with 1x500 mL of aq. potassium hydrosulfate (1M) and 1x500 mL of aq. sodium bicarbonate (5%) respectively. The organic layer was dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column and eluted with dichloromethane/methanol (100: 1). This resulted in Compound A3 as an oil. |
|
With triethylamine; In dichloromethane; at 25℃; for 1h;Inert atmosphere; |
Into a 2000-mL 3-necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen was placed a solution of 2-(2-hydroxyethoxy)ethan-l-ol (A2, 42.4 g,399.55 mmol, 1.00 equiv) in dichloromethane (1000 mL) and triethylamine (27.9 g, 275.72 mmol, 0.25 equiv). To the above was added p-toluenesulfonyl chloride (19.1 g, 100.18 mmol, 0.50 equiv). After stirring for 1 h at 25C, the resulting mixture was washed with lxSOO mL of aq. potassium hydrosulfate (1M) and lxSOO mL of aq. sodium bicarbonate (5%) respectively. The organic layer was dried over anhydrous sodium sulfate and concentrated under vacuum.The residue was applied onto a silica gel column and eluted with dichloromethane/methanol (100:1). This resulted in Compound A3 as an oil. |
| 22.7 g |
With pyridine; at 0 - 20℃;Inert atmosphere; |
Under the protection of nitrogen, to a 500ml three-necked flask were added 200 mL pyridine, 18.8 g BG01 (1.0eq), stirred and cooled down to 0C. 35.5g TsCl (1.0eq) was added in batches, stirred for 1h, and then slowly warmed up to room temperature, continuing to stir for 3-4h. After the completion of the reaction, the reaction liquid was poured into the ice-cold solution of dilute hydrochloric acid, with a solid being generated, which was extracted with ethyl acetate. The ethyl acetate layer was washed once with dilute hydrochloric acid, washed with saturated sodium bicarbonate and saturated brine, and dried over anhydrous Na2SO4. The solvents were evaporated off at reduced pressure, and chromatographed in a silica gel column to give 22.7g pure BG02. |
|
With triethylamine; In dichloromethane; at 0℃; for 2h;Inert atmosphere; |
General procedure: To a stirred solution of substituted ethylene glycol (1.0 eq.) and triethylamine (0.1 eq.) in DCM (500 mL) was added tosyl chloride (0.1 eq.) in DCM (10 vol.) dropwise at 0 C for 2 h under nitrogen atmosphere. Stirring was continued at RT for 20 h and then the reaction mixture was washed with saturated NH4CI solution, water, brine dried over anhydrous sodium sulphate and concentrated under vacuum to give the residue which was purified by combi flash using 10-15% ethyl acetate in n-hexane as eluent to afford title compounds (26ai-26a3 yield: 45-50%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping