| 58% |
With isopropylmagnesium chloride; In tetrahydrofuran; at -30 - -5℃; for 1h; |
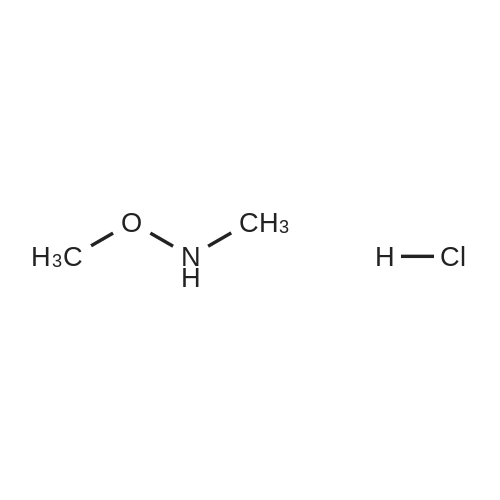
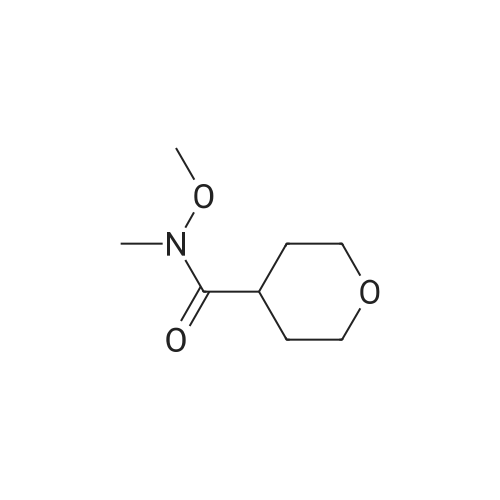
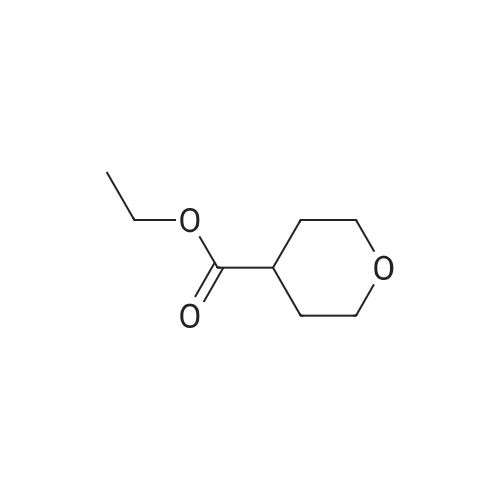
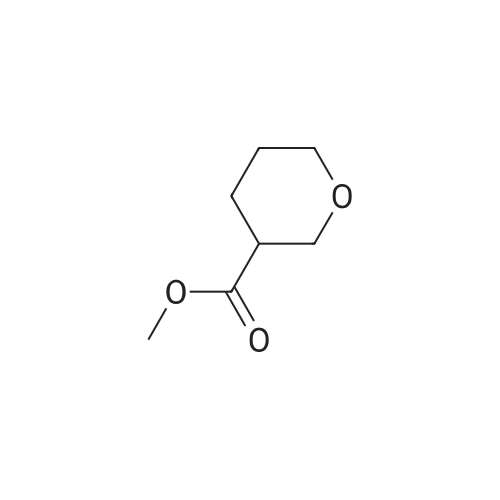
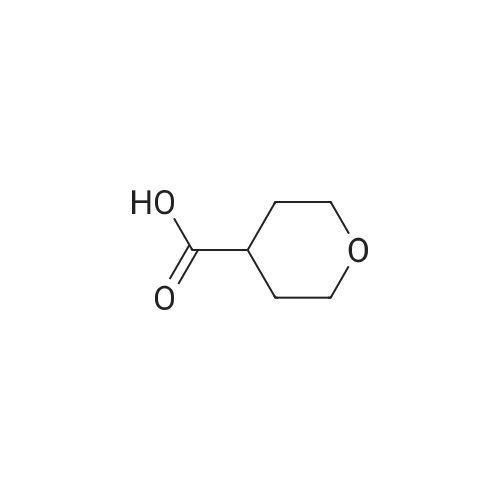
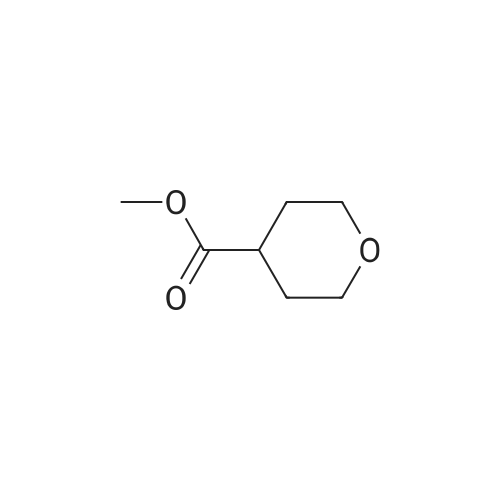
Step 1 Methyl tetrahydropyran-4-carboxylate (1.33 mL, 10.0 mmol) was dissolved in THF (20 mL), N,O-dimethylhydroxylamine hydrochloride (1.51 g, 15.5 mmol) was added thereto, and the mixture was stirred. A THF solution (15.0 mL, 30.0 mmol) of 2.0 mol/L isopropyl magnesium chloride was added dropwise to the reaction mixture at -30C under an argon atmosphere, and the mixture was stirred at -5C for 1 hour. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with brine and dried over anhydrous magnesium sulfate, and then the solvent was removed in vacuo. The resulting residue was purified by distillation under reduced pressure, whereby N-methoxy-N-methyl tetrahydropyran-4-carboxylic acid amide (1.00 g, 58%) was obtained. Boiling point: 125 to 129C/8.0 hPa 1H NMR (CDCl3, delta ppm): 1.57-1.66 (m, 2H), 1.77-1.93 (m, 2H), 2.85-2.94 (m, 1H), 3.18 (s, 3H), 3.44 (ddd, J = 2.4, 11.9, 11.9 Hz, 2H), 3.69 (s, 3H), 4.00 (ddd, J = 2.4, 11.9, 11.9 Hz, 2H) |
| 58% |
With isopropylmagnesium chloride; In tetrahydrofuran; at -30 - -5℃; for 1h;Inert atmosphere; |
Methyl tetrahydropyran-4-carboxylate (1.33 mL, 10.0 mmol) was dissolved in THF (20 mL), and N,O-dimethylhydroxylamine hydrochloride (1.51 g, 15.5 mmol) added thereto , then the mixture was stirred. Under an argon atmosphere, THF solution of isopropyl magnesium chloride (2.0 mol/L; 15.0 mL, 30.0 mmol) was added dropwise to the reaction mixture at -30C, and the mixture was stirred at -5C for 1 hour. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with brine and dried over anhydrous magnesium sulfate. The solvent was then evaporated under reduced pressure. The resulting residue was purified by distillation under reduced pressure to give N-methoxy-N-methyltetrahydropyran-4-carboxamide (1.00 g, 58%). Boiling point: 125-129 C / 8.0 hPa, 1H NMR (CDCl3, delta ppm): 1.57-1.66 (m, 2H), 1.77-1.93 (m, 2H), 2.85-2.94 (m, 1H), 3.18 (s, 3H), 3.44 (ddd, J = 2.4, 11.9, 11.9 Hz, 2H), 3.69 (s, 3H), 4.00 (ddd, J = 2.4, 11.9, 11.9 Hz, 2H). |
|
With isopropylmagnesium chloride; In tetrahydrofuran; at -20℃;Inert atmosphere; |
Add 2 M isopropylmagnesium chloride in tetrahydrofuran (520.22 mL, 3.0 eq) to a mixture of methyl tetrahydro-2H-pyran-4-carboxylate (46.30 mL, 346.81 mmol) and Nu,Omicron-dimethylhydroxylamine hydrochloride (52.44 g, 1.6 eq) in tetrahydrofuran (2.43 L) during 15 minutes at -20C under nitrogen. After 30 min, add saturated aqueous ammonium chloride (400 mL) to the reaction at -20C. Extract the aqueous solution with methyl tert-butyl ether (250 mL x 3). Wash the combined organics with saturated aqueous sodium chloride. Dry over anhydrous magnesium sulfate and concentrate in vacuo. Add dichloromethane (500 mL), filter through Celite and concentrate in vacuo. Add tetrahydrofuran (700 mL), then add 3 M methyl magnesium chloride intetrahydrofuran (231.21 mL, 2.0 eq) dropwise over 15 minutes at 7C. After 40 minutes, add saturated aqueous ammonium chloride (250 mL) to the reaction. Extract the aqueous solution with methyl tert-butyl ether (250 mL x 2). Dry over anhydrous magnesium sulfate and concentrate in vacuo. Purify by silica gel chromatography, eluting with 2: 1 hexanes: ethyl acetate to 1 : 1 hexanes: ethyl acetate, to give 1 -(tetrahydro-pyran-4-yl)- ethanone (33.18 g, 75%). 'H NMR (300 MHz, DMSO-d6) delta 3.98 (m, 2H), 3.42 (m, 2H), 2.52 (m, 1H), 2.15 (s, 3H), 1.74 (m, 4H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping