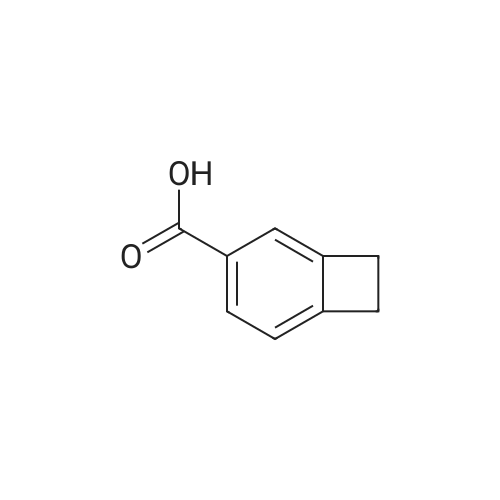
| 51.1% |
With bromine; iodine; acetic acid; In water; at 0 - 20℃; |
Br2 (1.10 g, 6.88 mmol) dropwise to a mixture of 1, 2-dihydrocyclobutabenzene (scheme 8-46 compound S1, 650 mg, 6.25 mmol) and I2 (14 mg, 0.056 mmol) in AcOH/H2O (8 mL/ 0.4 mL) at 0 oC for 20 min. The reaction was stirred at room temperature overnight. The mixture was poured into water and extracted with petroleum ether (50 mL). The organic layer was washed with aqeuous Na2CO3 solution and water, dried over anhydrous Na2SO4, and concentrated. The residue was purified by column chromatography on silica gel eluted with petroleum ether to afford scheme 8-46 compound S2 (490 mg, 51.1 %) as a light yellow oil. |
| 48% |
With bromine; iodine; In acetic acid; at 20℃; |
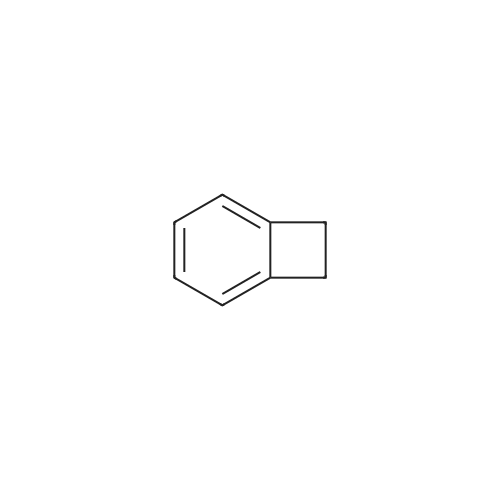
The synthesis of a tetracene brominated in the 2-position is shown by way of example in the following illustration: In a first step, alpha-chloro-o-xylene 1 was subjected to pyrolysis at approximately 800 C. and 0.5 mbar. Benzocyclobutene 2 was obtained in a 45% yield. The selective bromination thereof was carried out by treating <strong>[694-87-1]benzocyclobutene</strong>, dissolved in acetic acid, with a mixture of bromine and iodine at room temperature, resulting in 4-bromo<strong>[694-87-1]benzocyclobutene</strong> 3. Dissolving in toluene and heating with a slight molar excess of 1,4-dihydro-1,4-epoxynaphthalene 4 at 220 C. for 20 hours resulted in an 80% yield of a pure endo/exo mixture of the Diels-Alder addition product 5, a colorless crystalline material. This material was heated at reflux in acetic anhydride in the presence of concentrated hydrochloric acid, thus forming 9-bromo-6,11-dihydrotetracene 6. The Yamamoto coupling then resulted in 2-(5,12-dihydrotetracene-2-yl)-5,12-dihydrotetracene 7. The coupling reaction was carried out in an approximately 80% yield in a mixture of dimethylformamide and toluene at 80 C., using bis(cyclooctadienyl) nickel(0) in stoichiometric quantities. After recrystallization from o-dichlorobenzene, compound 7 was dehydrogenated by treatment with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) in boiling o-xylene. After purification by repeated vacuum sublimation, orange-red crystals of 2-(tetracene-2-yl) tetracene 8 were obtained in a yield of 75%. All intermediate products were characterized by 1H and 13C NMR spectroscopy and mass spectroscopy. Compound 8 was characterized by UV-visible spectroscopy. |
|
With bromine; In water; at 20℃;Cooling with ice; |
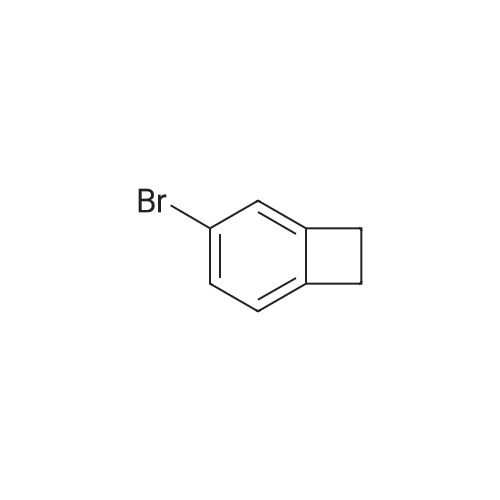
Step 1 3-Bromo-bicyclo[4.2.0]octa-1 (6),2,4-triene; Bicyclo[4.2.0]octa-1(6),2,4-triene 5a (7.9 g,76 mmol) was dissolved in 80 mL of water at room temperature. Upon cooling by an ice-water, 3.9 mL of bromine was added dropwise. Upon completion of the addition, the ice-water bath was removed and the reaction mixture was warmed up to room temperature and stirred overnight. The reaction was monitored by TLC until the disappearance of the starting materials. The mixture was diluted with 50 mL of n-hexane and sodium sulfite (3 g, 23.8 mmol) was added. Upon completion of the addition, the mixture was stirred at room temperature for 30 minutes. Then the separated organic layer was dried over anhydrous sodium sulfate, filtered to remove the drying agent and concentrated under reduced pressure to obtain the title compound 3-bromo-bicyclo[4.2.0]octa-1(6),2,4-triene 5b (13.53 g) as a colourless oil, which was directly used in the next step. MS m/z (ESI): 181.8 [M-1] |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping