| 100% |
|
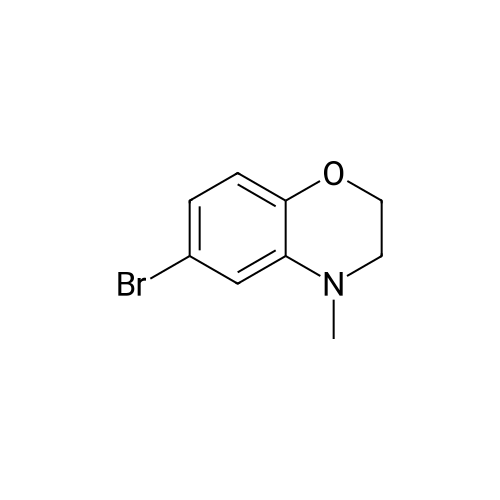
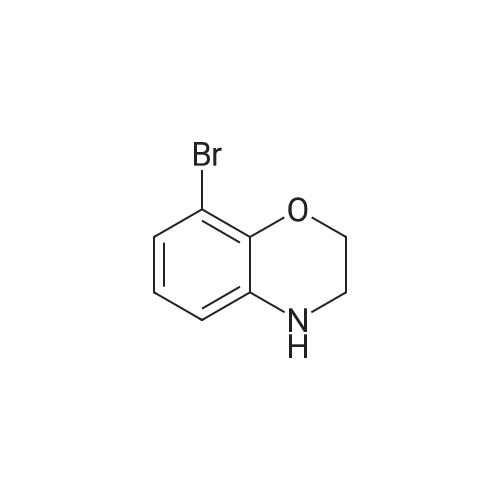
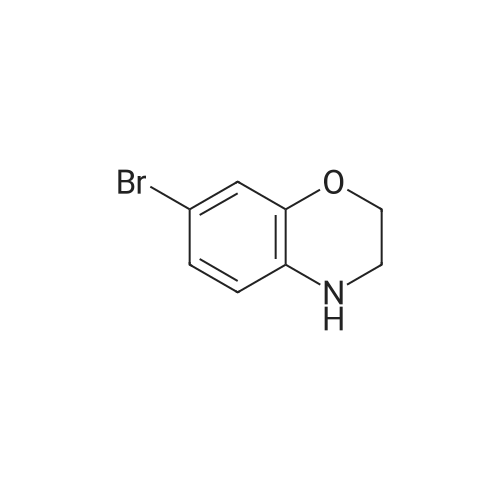
Borane-THF (13.2 mL, IM solution in THF, 13.2 mmol) was added portionwise to Intermediate 5 (2.0 g, 8.0 mmol) in THF (50 mL) at r.t. The resulting solution was stirred at r.t. for 10 minutes, heated to reflux for 1 h and then allowed to cool to r.t. The reaction mixture was cooled to 00C and quenched with water (20 mL) and aqueous 2N NaOH (20 mL). The solvent was removed in vacuo and the resulting mixture diluted with water (100 mL). The aqueous fraction was extracted with EtOAc (100 mL), washed with brine (100 mL), dried (MgSO4), filtered and concentrated in vacuo to yield the title compound (2 g, quantitative) as a brown oil. deltaH (DMSO-d6) 6.68 (3H, m), 4.25-4.18 (2H, m), 3.81 (IH, br. s), 3.44-3.36 (2H, m). |
| 100% |
|
To a stirred solution of Intermediate 32 (2.0 g, 8.0 mmol) in TetaF (50 mL) was added borane-TetaF (13.2 mL, IM solution in TetaF, 13.2 mmol) portionwise. The reaction mixture was stirred at r.t. for 10 minutes, then heated to reflux for 1 h and allowed to cool to r.t. The reaction mixture was cooled to 00C and quenched with water (20 mL), then 2M aqueous NaOH (20 mL), and concentrated in vacuo. Water (100 mL) and EtOAc (100 mL) were added and the layers separated. The organic fraction was washed with <n="105"/>brine (100 mL), dried (MgSO4), filtered and concentrated in vacuo to give the title compound (2 g, quantitative) as a brown oil. deltaH (DMSOd6) 6.68 (3 H, m), 4.25-4.18 (2H, m), 3.81 (IH, br. s), 3.44-3.36 (2H, m). |
| 93% |
With dimethylsulfide borane complex; In tetrahydrofuran; for 3h;Inert atmosphere; Reflux; |
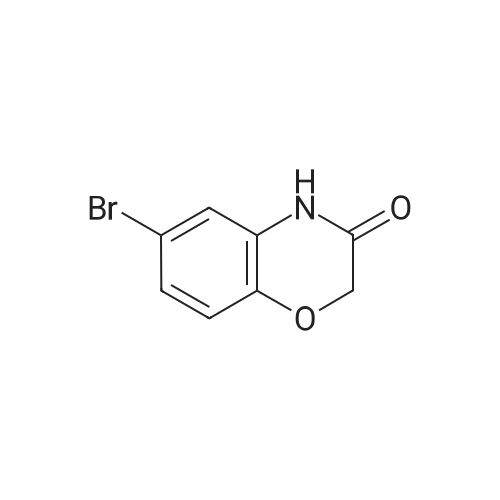
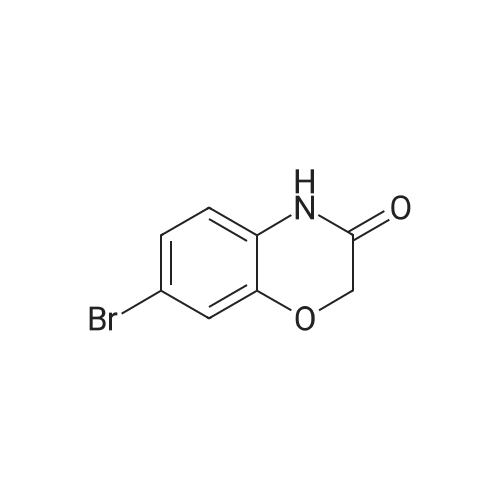
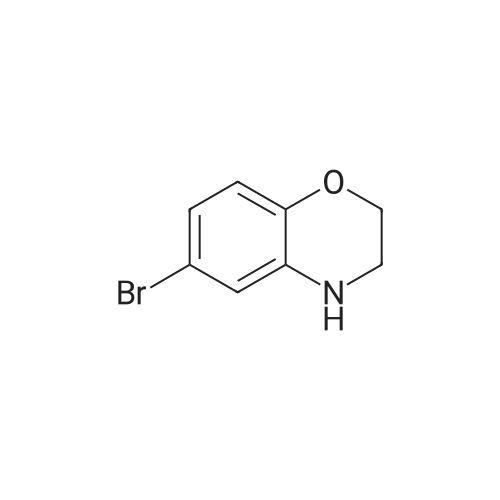
To a solution of 6-bromo-2H-benzo[b][1,4]oxazin-3(4H)-one (400 mg, 1.75 mmol) in anhydrous tetrahydrofuran (3 mL) under a nitrogen atmosphere was added a 1M solution of borane dimethyl sulfide complex in tetrahydrofuran (7.0 mL, 7.0 mmol) slowly. The resulting solution was refluxed for 3 hours. Then, the reaction mixture was cooled to ambient temperature and carefully quenched with the addition of methanol (10 mL). Next, the reaction mixture was heated to reflux for 10 minutes, and then the reaction mixture was cooled and concentrated in vacuo to provide a residue. The residue was redissolved in ethyl acetate, washed with water, brine, dried with sodium sulfate, filtered and concentrated in vacuo to yield title compound. The title compound was used in Part IV below without purification. (350 mg, 93% yield) ESI m/z 214.03, 216.0 (M+H). |
| 88% |
|
Example 48; lambda/-{2-chloro-5-[4-(phenylmethyl)-3,4-dihydro-2H-l,4-benzoxazin-6-yl]-3- pyridinyl}benzenesulfonamide; a) 6-bromo-3 ,4-dihy dro-2H- 1 ,4-benzoxazine; 6-Bromo-2H-l,4-benzoxazin-3(4H)-one (2.085 g, 9.143 mmol) was suspended in dry TetaF (20 mL) and placed under nitrogen with stirring and to this was added 1 M BH3-THF complex (3.143 g, 36.572 mmol, 36.52 mL) over 5 minutes. Addition causes the reaction to become homogeneous. After 70 minutes, the reaction was cooled to O0C and made acidic by addition of 3N HCl (109 mL). Addition of acid causes vigorous bubbling. After the addition was completed, the reaction was refluxed for 10 minutes and then cooled and made basic by addition of 6N NaOH. The basified reaction was extracted with EtOAc (3 x 100 mL), dried over Na2SO4, filtered, and the concentrated filtrate was purified by flash chromatography on silica gel (.00% CH2Cl2) to give the title compound (1.713 g, 88%) as a pale yellow oil. MS(ES)+ m/e 214 [M+H]. |
| 87% |
With borane-THF; In tetrahydrofuran;Inert atmosphere; Cooling with ice; Reflux; |
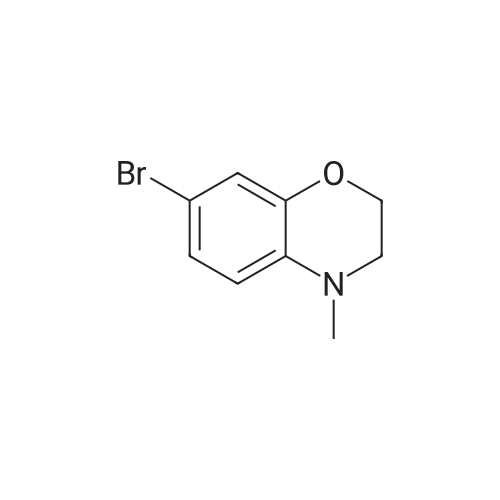
[Synthesis of Compound E4] (0133) Compound E3 (1.0 g, 4.4 mmol, 1 Eq) was dissolved in THF (20 mL) in an argon atmosphere, and placed on an ice bath. A THF solution of 1 M borane-THF complex (7.0 mL, 7.0 mmol, 1.6 Eq) was added and the combination was stirred overnight while heating and refluxing. The reaction system was returned to room temperature, methanol was added in small amounts, and the solvent was removed under reduced pressure. The reaction system was then diluted by ethyl acetate, and the organic layer was washed by saturated sodium hydrogen carbonate aqueous solution and saturated saline, dried by anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The residue obtained was purified by medium-pressure silica gel chromatography (eluent: dichloromethane/methanol=98/2), and the target compound E4 (817 mg, 87%) was obtained as a colorless liquid. (0134) 1H NMR (CDCl3): delta 6.71 (dd, J=2.3 Hz, 8.4 Hz, 1H), 6.68 (d, J=2.3 Hz, 1H), 6.62 (d, J=8.4 Hz, 1H), 4.20 (t, J=4.4 Hz, 2H), 3.80 (br s, 1H), 3.38 (t, J=4.4 Hz, 2H).; 13C NMR (CDCl3): delta 143.1 (C), 135.2 (C), 121.2 (CH), 118.1 (CH), 117.7 (CH), 113.2 (C), 65.1 (CH2), 40.6 (CH2).; HRMS-ESI (m/z): [M+H]+ calcd for C8H9BrNO: 213.98620. found: 213.98626 (-0.1 mDa, -0.3 ppm). |
|
With borane-THF; In tetrahydrofuran; for 14h;Reflux; |
Step II: To a stirred solution of 6-bromo-2H-1,4-benzoxazin-3(4H)-one (10 g, 43.85 mmol) in THF (25 ml) was added 1.0 M borane-tetrahydrofuran complex in THF (153.5 ml, 153.48 mmol) and refluxed for 14 h. The reaction mixture was cooled to room temperature, quenched with methanol (50 ml) and concentrated under reduced pressure. The resulting residue was taken in ethyl acetate (100 ml), washed with aqueous saturated sodium bicarbonate solution (100 ml), water (100 ml), brine (100 ml), dried over sodium sulphate, concentrated and purified by silica gel column chromatography (5% ethyl acetate in hexane) to provide 6-bromo-3,4-dihydro-2H- benzop^oxazine (8.5 g). |
| 3.2 g |
With borane-THF; In tetrahydrofuran; at 0℃; for 3h;Reflux; |
6-bromo-3,4-dihydro-2H-benzo[6] [l,4]oxazine (2): To a stirred solution of 6- bromo-2H-benzo[6][l,4]oxazin-3(4H)-one (7 g, 30.8 mmol) in THF (20 mL) was added 1M borane in THF (15.41 mL) at 0 C and stirred for 3 h at reflux temperature. After completion of the reaction, methanol (2 mL) was added to the reaction mixture at 0 C and stirred for 2 h at reflux. After completion of the reaction, cone. HC1 (2 mL) was added to reaction mixture at 0 C and again stirred for 2 h at reflux. The reaction mixture was then neutralized with 2N NaOH solution at 0 C and extracted with ethyl acetate. The combined organic layer was washed with water and brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude compound was purified by column chromatography (100-200 mesh silica gel, 15% ethyl acetate in pet. ether as eluent) to afford 2 (3.2 g, 49% yield) as a brown solid. MS m/z (M+H): 214.4 |
|
With borane-THF; In tetrahydrofuran; at 0℃;Reflux; |
Compound 7-2 (22 g, 0.1 mol) was dissolved in tetrahydrofuran (200 ml) and a solution of BH3 / tetrahydrofuran (1.0 M, 200 ml) was added dropwise at 0 C. The mixture was then stirred under reflux overnight. Then slowly at 0 reaction was quenched with methanol. The mixture was concentrated to give a crude product was used directly in the next step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping