|
With triethylamine; In chloroform; at 0℃; for 1.5h; |
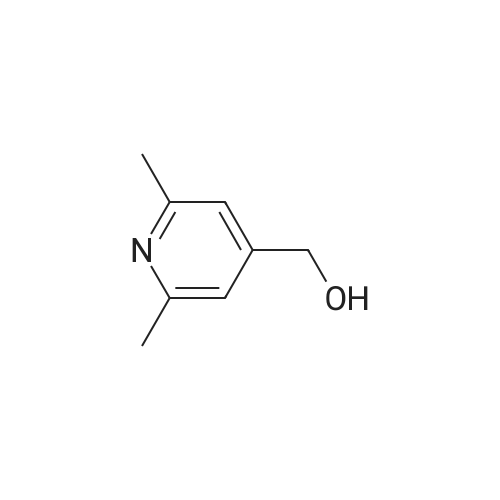
Example BM; To a stirred solution of 2-chloro-4-pyridinemethanol (123 mg, 1 mmol) in chloroform (10 ml) at 0 C. (ice bath), was added triethylamine (210 mul, 1.5 mmol) and methanesulfonyl chloride (90 mul, 1.2 mmol). After stirring for 1.5 hr., the mixture was diluted with dichloromethane, washed with aqueous saturated sodium bicarbonate solution, dried with anhydrous magnesium sulfate, filtered, and evaporated in vacuo. The residue was further dried in high vacuo and mixed with 3-[3-(5-isopropyl-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidine-4-carbonyl)-5-methyl-phenyl]-acrylonitrile (323 mg, 1 mmol), anhydrous powdered potassium carbonate (138 mg, 1 mmol), lithium iodide (134 mg, 1 mmol). Anhydrous DMF (5 ml) was then added into the mixture and stirred for overnight at room temperature. The mixture was evaporated in vacuo. The residue was dissolved in methanol-dichloromethane (1:9), filtered through celite pad, and the filtrate was evaporated in vacuo and the residue was purified by silica gel column chromatography (eluent, EA:hexane (1:1)) to afford 186 mg (43%) of a white solid. m.p. 173-174 C.; 1H-NMR (200 MHz, CDCl3/CD3OD) delta 1.09(3H, d, J=6.8 Hz), 1.20 (3H, d, J=6.8 Hz), 2.26 (1H, m), 2.37 (6H, s), 4.54 (1H, d, J=16.6 Hz), 4.89 (1H, d, J=16.6 Hz), 6.02 (1H, d, J=16.6 Hz), 6.81-6.84 (2H, m), 7.38 (1H, d, J=16.6 Hz), 7.51 (1H, s), 7.54 (1H, s), 7.68 (1H, s), 8.18 (1H, dd, J=1.4 Hz, 3.4 Hz); m/z (EI) 428(M+). |
|
With triethylamine; In dichloromethane; at 0 - 20℃; |
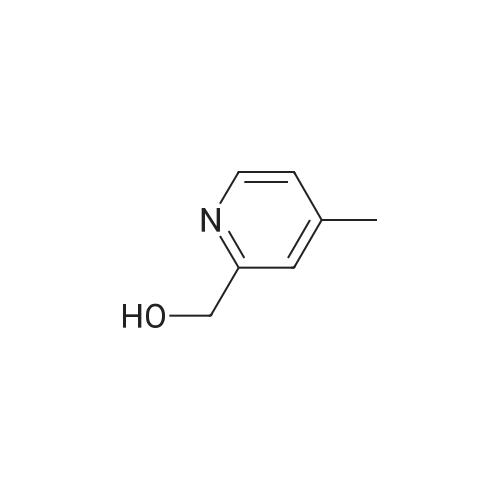
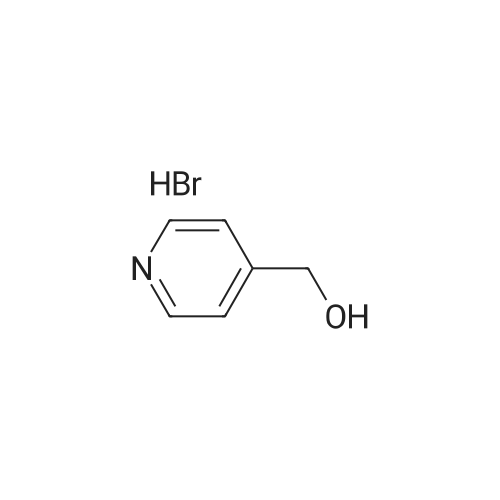
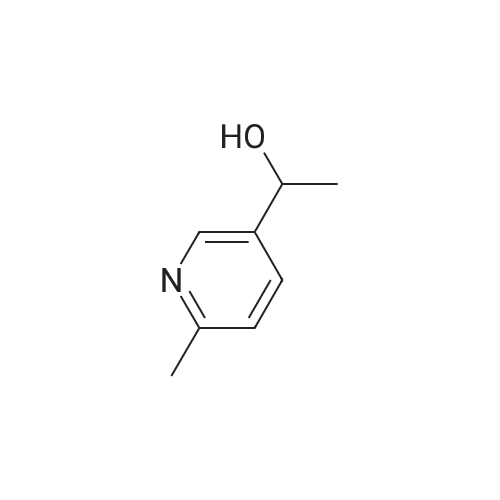
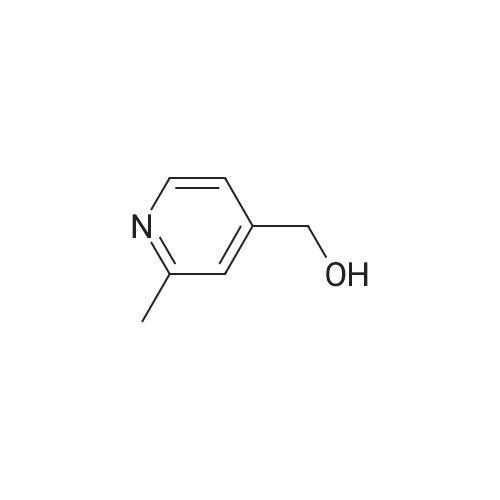
The mesylate of <strong>[105250-16-6](2-methylpyridin-4-yl)methanol</strong> (100 mg) was prepared by reaction with methanesulfonyl chloride (1 .1 eq) and triethylamine (2 eq) in DCM at 0 C, with warming to ambient temperature. N-[1 -(Fluoromethyl)cyclopropyl]-3-[(1 -methylpyrazol-4- yl)methyl]-2,4-dioxo-1 1H-quinazoline-6-sulfonamide (100 mg, 0.260 mmol), the crude mesylate (58 mg, 0.286 mmol) and potassium carbonate (43 mg, 0.312 mmol) in DMF was conventionally heated to 70 C for 4 h. Usual work-up afforded the desired product (46 mg, 0.090 mmol, 35%) as a white powder. |
|
With triethylamine; In ethyl acetate; for 4h; |
Alcohol S6 (0.040 g, 0.325 mmol) was added to an oven dried 20mL vial, followed by ethyl acetate (10 mL). Next, mesyl chloride (0.030 mL, 0.390 mmol) was added, followed by NEt3(0.054 mL, 0.390 mmol). The reaction was stirred for 4 h, after which time a sample aliquot was taken from the reaction,dissolved in 1 mL HPLC grade MeCN, and analyzed with LC-MS to confirm the completion of the reaction. The reaction was diluted with EtOAc (50 mL), and washed with water (10 mL) and brine before being condensed, yielding the titlecompound as a light yellow oil. The compound was used directly in the next step of the reaction without further purificationor analysis. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping