| 81% |
With bromine; triphenylphosphine; In dichloromethane; at 0 - 5℃; for 4h;Cooling with ice; |
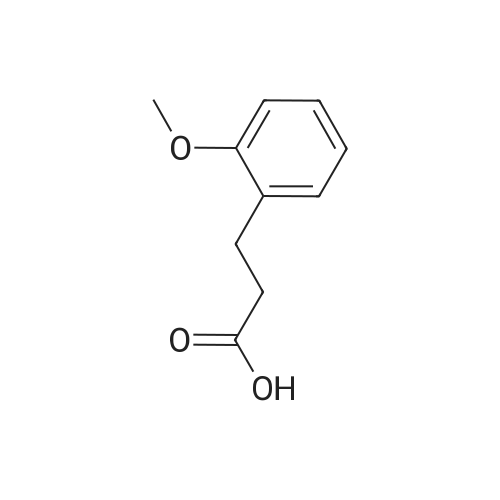
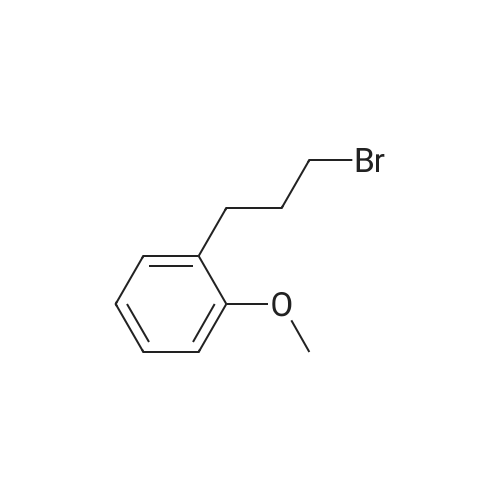
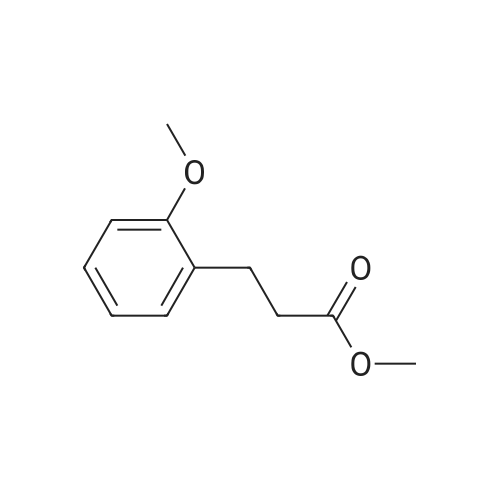
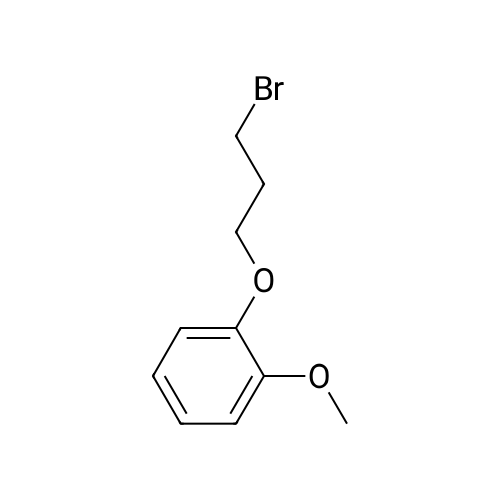
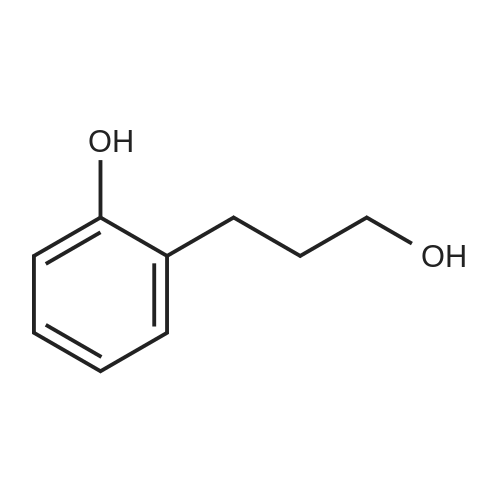
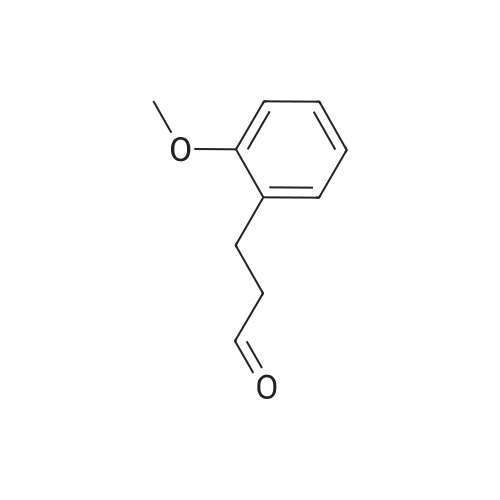
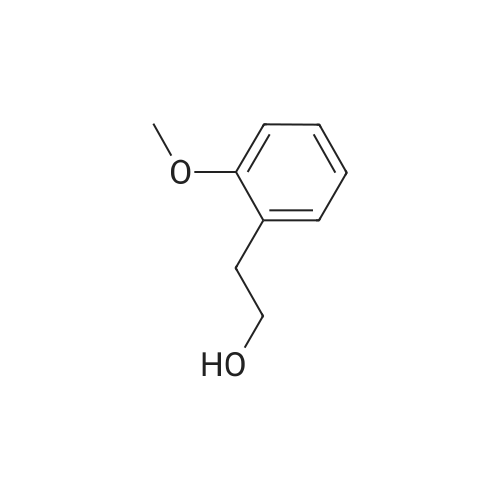
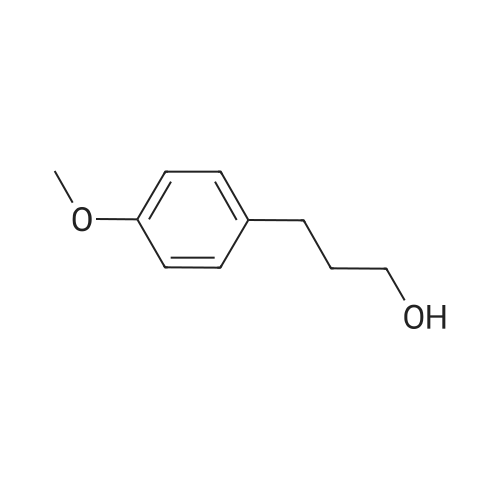
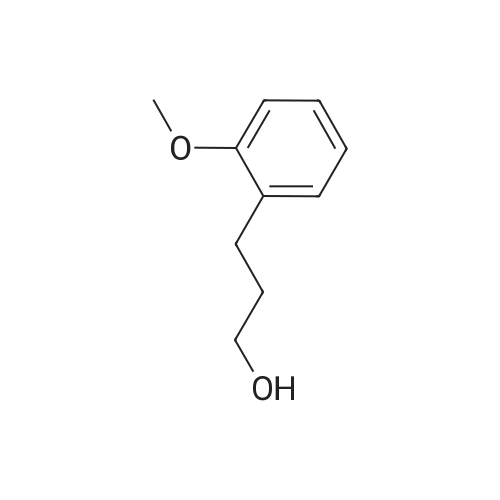
3-(2-Methoxyphenyl)propan-1-ol (1.6 g, 9.7 mmol) was dissolved in DCM (43 mL) to which was added triphenylphosphine (2.7 g, 0.01 mol). The solution was cooled in an ice bath and bromine (1.6 g, 0.01 mol) was added dropwise over 4 h whilst the reaction was maintained at 0-5C. The reaction was quenched with a saturated aqueous solution of NaHCO3 (15 mL), the DCM layer removed and the aqueous layer extracted with more DCM (50 mL3). The combined organic layers were dried (Na2SO4) and concentrated to give a yellow solid. The solid was loaded onto silica (1 g) and purified by chromatography (silica, 24 g) eluting with hexane (3CV), 0-100% EtOAc in hexane (40CV) and EtOAc (CV). A clear oil was obtained (1.81 g, 81%). 1H NMR (CDCl3, 600 MHz) δ 7.19 (1H, td, J 1.8, 7.8 Hz), 7.14 (1H, dd, J 1.2, 7.2 Hz), 6.87 (1H, td, J 0.6, 7.2 Hz), 6.84 (1H, d, J 8.4 Hz), 8.31 (3H, s), 3.39 (2H, t, J 6.6 Hz), 2.75 (2H, t, J 7.2 Hz), 2.13 (2H, t, J 7.2 Hz). |
| 81% |
With bromine; triphenylphosphine; In dichloromethane; at 0 - 5℃; for 4h;Cooling with ice; |
3-(2-Methoxyphenyl)propan-1-ol (1.6 g, 9.7 mmol) was dissolved in DCM (43 mL) to which was added triphenylphosphine (2.7 g, 0.01 mol). The solution was cooled in an ice bath and bromine (1.6 g, 0.01 mol) was added dropwise over 4 h whilst the reaction was maintained at 0-5C. The reaction was quenched with a saturated aqueous solution of NaHCO3 (15 mL), the DCM layer removed and the aqueous layer extracted with more DCM (50 mL3). The combined organic layers were dried (Na2SO4) and concentrated to give a yellow solid. The solid was loaded onto silica (1 g) and purified by chromatography (silica, 24 g) eluting with hexane (3CV), 0-100% EtOAc in hexane (40CV) and EtOAc (CV). A clear oil was obtained (1.81 g, 81%). 1H NMR (CDCl3, 600 MHz) δ 7.19 (1H, td, J 1.8, 7.8 Hz), 7.14 (1H, dd, J 1.2, 7.2 Hz), 6.87 (1H, td, J 0.6, 7.2 Hz), 6.84 (1H, d, J 8.4 Hz), 8.31 (3H, s), 3.39 (2H, t, J 6.6 Hz), 2.75 (2H, t, J 7.2 Hz), 2.13 (2H, t, J 7.2 Hz). |
|
With N-Bromosuccinimide; triphenylphosphine; In dichloromethane; at 20℃; for 16h;Ice-cooling; |
(17-2) Synthesis of 1-(3-bromopropyl)-2-methoxybenzene (compound 17-2) Compound 17-1 (2.00 g) was dissolved in methylene chloride (50 ml), triphenylphosphine (3.58 g) and N-bromosuccinimide (2.40 g) were added under ice-cooling, and the mixture was stirred under ice-cooling for 1 hr, and further at room temperature for 15 hr. The reaction mixture was washed with water and saturated brine, and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure. Diethyl ether (100 ml) was added, and the precipitated triphenylphosphine oxide was filtered off. The concentrate of the filtrate was purified by silica gel column chromatography (hexane alone) to give the object product (2.24 g) as a pale-brown oil. 1H-NMR(CDCl3) δ (ppm): 2.11-2.18(2H, m), 2.76(2H, t, J=7.3Hz), 3.40(2H, t, J=6.9Hz), 3.82(3H, s), 6.83-6.90(2H, m), 7.13-7.22 (2H, m). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping