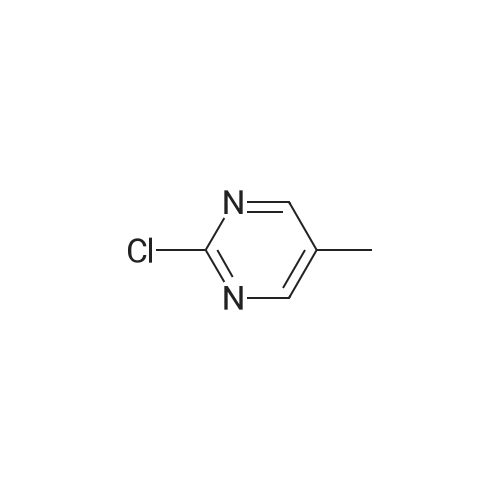
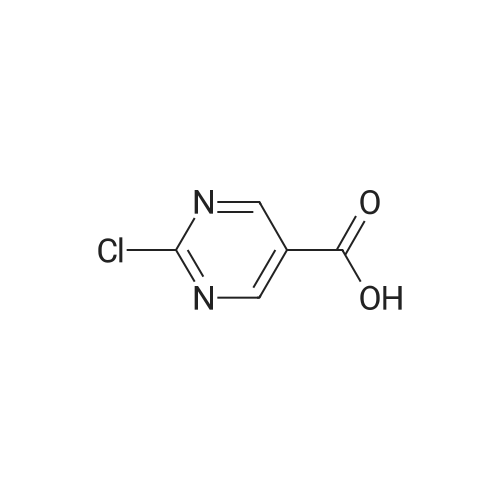
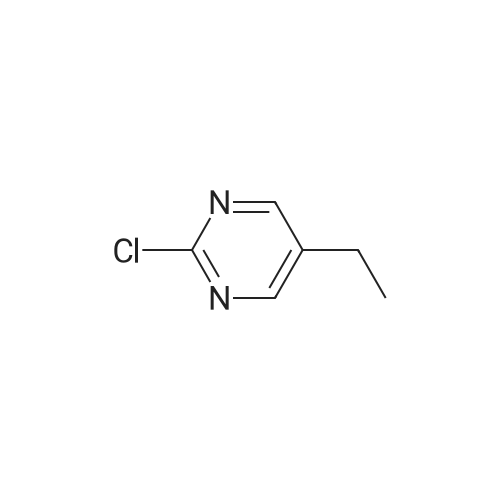
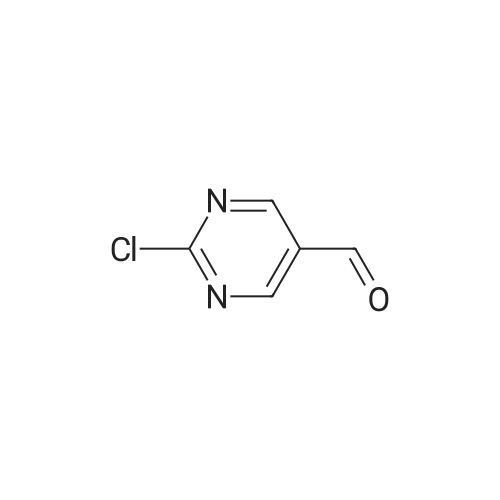
| 66% |
With sodium hydride; In N,N-dimethyl-formamide; at 0 - 20℃; for 0.833333h; |
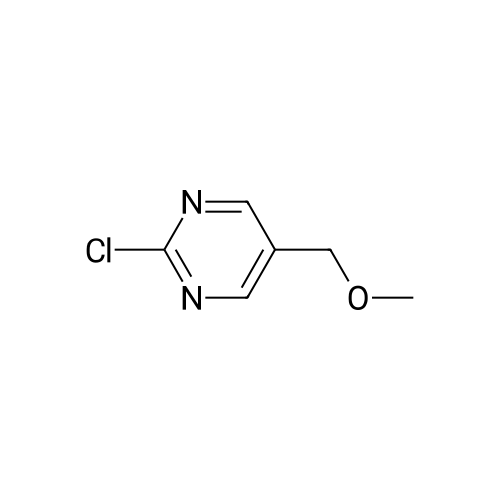
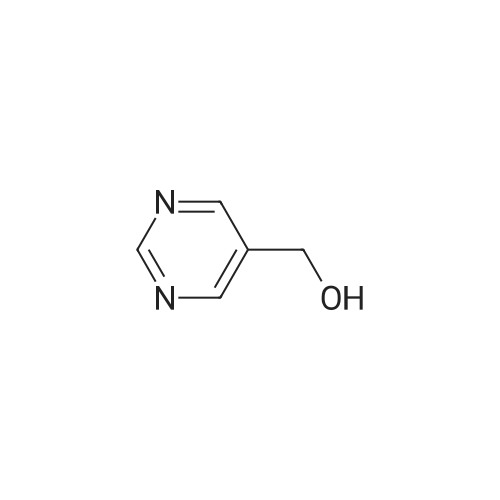
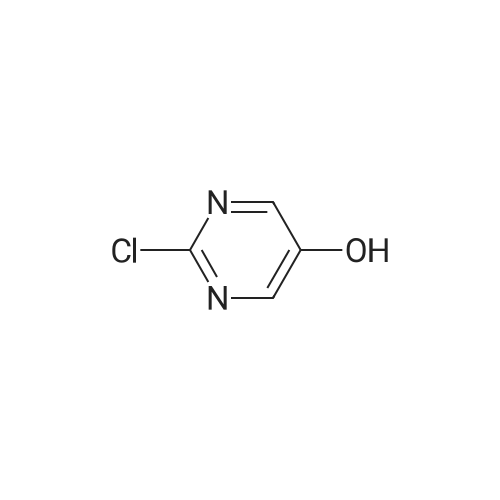
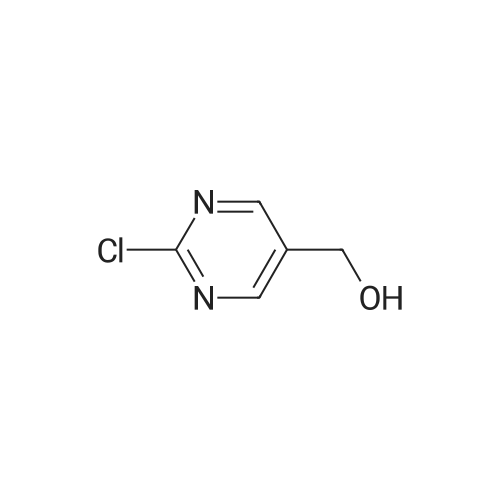
To a solution of <strong>[1046816-75-4]2-chloro-5-hydroxymethyl-pyrimidine</strong> (9.0 g, 62 mmol) in 70 ml of anhydrous DMF was added methyl iodide (6 eq. 370 mmol, 23 ml). The mixture was cooled to 0°C, then NaH (2.6 lg, 1.05 eq.) was added in portions over 5 mins. The resulting mixture was stirred 25 min. at 0°C, then 25 min. at rt. The reaction mixture was then cooled in ice bath, and quenched by addition of saturated NH4C1 aq. solution (200 ml ), extracted with ether (150 ml x 3). The combined organic layers were washed by brine, dried over Na2S04, filtered and concentrated in vacuo. The residue was purified by ISCO column (330 g of silica gel) using ethyl acetate in hexane (0-90percent ethyl acetate, 2500 ml, then 1000 ml of ethyl acetate) to give 6.5g (66percent) of the title compound: MS (ESI) mlz 159.2 (M+H); 1H NMR (500 MHz, CDC13) delta 8.60 (s, 2H), 4.48 (s, 2H), 3.45 (s, 3H). |
| 66% |
With sodium hydride; In N,N-dimethyl-formamide; at 0 - 20℃; for 0.916667h; |
To a solution of <strong>[1046816-75-4]2-chloro-5-hydroxymethyl-pyrimidine</strong> (9.0 g, 62 mmol) in 70 ml of anhydrous DMF was added methyl iodide (6 eq. 370 mmol, 23 ml). The mixture was cooled to 0°C, then NaH (2.6 lg, 1.05 eq.) was added in portions over 5 mins. The resulting mixture was stirred 25 min. at 0°C, then 25 min. at rt. The reaction mixture was then cooled in ice bath, and quenched by addition of saturated NH4CI aq. solution (200 ml ), extracted with ether (150 ml x 3). The combined organic layers were washed by brine, dried over Na2S04, filtered and concentrated in vacuo. The residue was purified by ISCO column (330 g of silica gel) using ethyl acetate in hexane (0-90percent ethyl acetate, 2500 ml, then 1000 ml of ethyl acetate) to give 6.5g (66percent) of the title compound: MS (ESI) mlz 159.2 (M+H); NMR (500 MHz, CDC13) delta 8.60 (s, 2H), 4.48 (s, 2H), 3.45 (s, 3H). |
| 66% |
With sodium hydride; In N,N-dimethyl-formamide; at 0 - 20℃; for 0.833333h; |
To a solution of<strong>[1046816-75-4]2-chloro-5-hydroxymethyl-pyrimidine</strong> (9.0 g, 62 mmol) in 70 ml ofanhydrous DMF was added methyl iodide (6 eq. 370 mmol, 23 ml). The mixture was cooled to0°C, then NaH (2.61g, 1.05 eq.) was added in portions over 5 mins. The resulting mixture wasstirred 25 min. at 0°C, then 25 min. at rt. The reaction mixture was then cooled in ice bath, and10 quenched by addition of saturated NH4Cl aq. solution (200 ml ), extracted with ether (150 ml x3). The combined organic layers were washed by brine, dried over Na2S04, filtered andconcentrated in vacuo. The residue was purified by ISCO column (330 g of silica gel) usingethyl acetate in hexane (0-90percent ethyl acetate, 2500 ml, then 1000 ml of ethyl acetate) to give 6.5g(66percent) of the title compound: 1H NMR (500 MHz, CDCh) 8 8.60 (s, 2H), 4.48 (s, 2H), 3.45 (s,15 3H). MS ESI m/z 159.2 (M+H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping